Markownikoff's Rule: Alkene Addition & Carbocations
advertisement

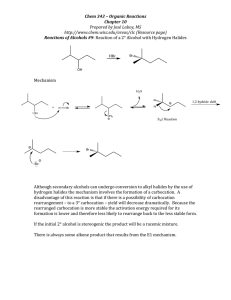
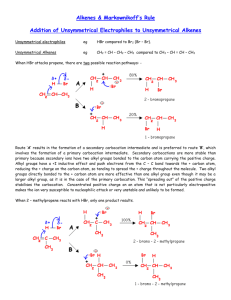
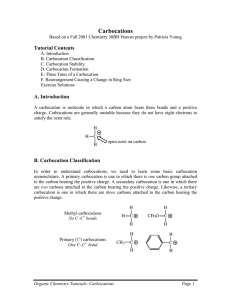
Markownikoff’s Rule Markownikoff’s Rule “When a hydrogen halide reacts with an unsymmetrical alkene the hydrogen of the hydrogen halide attaches itself to the carbon of the alkene bearing the larger number of hydrogens and smaller number of carbons" Addition reactions of unsymmetrical alkenes Addition of HBr to propene CH3CH=CH2: • H–Br can add in two different ways • Two products are possible: CH3CH=CH2 H–Br Contains more H atoms CH3CH=CH2 Br–H CH3CH2–CH2Br CH3CHBr–CH3 Minor product Major product It’s all controlled by carbocations H Primary carbocation One C joined to C+ C H H H C C H CH3 C H d+ Br d- CH3 Secondary carbocation Two C joined to C+ H H H C H C H H Br H CH3 Br H H H C C C Br H H Minor product H H H H H C C C H Br H Major product H Types of carbocation Primary carbocation • Least stable One carbon chains attached to C+ Secondary carbocation: • Two carbon chains attached to C+ Tertiary carbocation: • Three carbon chains attached to C+ Most stable Reasons for carbocation stability Least stable Electron-donating ability of alkyl groups. • • Each alkyl group pushes electrons towards C+ charge of carbocation. Primary Positive charge is spread over alkyl groups The more alkyl groups attached to the C+ • The more the positive charge is spread out • The greater the stability of the carbocation Secondary Most stable Tertiary