Formation of Alkenes from Alcohols by Dehydration
advertisement

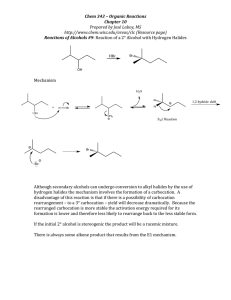
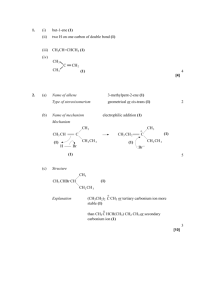
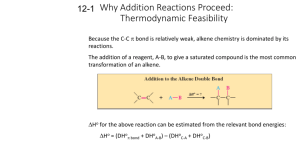
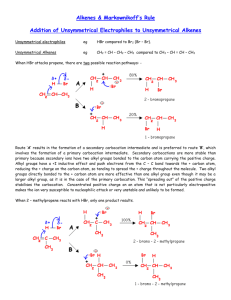
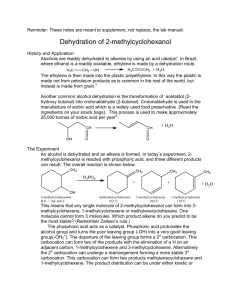
Formation of Alkenes from Alcohols by Dehydration - ELIMINATION Pass the vapour of the alcohol over heated aluminium oxide, or mix the alcohol with concentrated sulphuric acid or concentrated phosphoric acid and heat to 180oC. The alcohol is dehydrated. In effect, an H atom and an – OH group are removed from adjacent carbon atoms, leaving a double bond. This process of removing atom or atoms from adjacent carbon atoms leaving a double bond is the exact opposite of addition and is called elimination. The first step is the protonation of the OH group followed by the loss of a water molecule leaving a carbocation. This carbocation then loses a proton to produce the alkene. So a proton is regenerated and the reaction is therefore acid catalysed. The mechanism of this reaction IS required. Cyclohexanol forms cyclohexene and ethanol forms ethene. + H+ CH3 CH2 OH CH2 = CH2 + H2O Some alcohols can form more than one alkene. Butan2ol forms but1ene and but2ene because, during the second stage where a proton leaves the carbocation to form a double bond, the proton can leave from one of two carbon atoms and consequently form one of two isomers of butene. Forms but–1–ene forms but–2–ene Of the three alcohols with the formula C4 H9 OH, the tertiary alcohol, 2–methylpropan–2–ol has the fastest rate of dehydration, with butan–2–ol next and, finally butan–1–ol last. This is because the tertiary alcohol produces a tertiary carbocation intermediate that is more stable than the secondary which is more stable than the primary carbocation intermediate formed by butan–1–ol. 1