Resonance Structures Chemistry Worksheet
advertisement

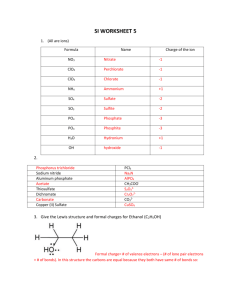
CW2: Resonance Structures Name: __________________________________________________ TP: ____________ Honors Chemistry Date: 2/3/14 CLASSROOM COPY: DO NOT WRITE ON!!! 1. Write Lewis Structures for the following. Show all resonance structures where applicable. NO2-, NO3-, N2O4 (N2O4 exists as O2N NO2) 2. Write Lewis Structures for the following. Show all resonance structures where applicable. OCN-, SCN-, N3- (Carbon is the central atom in OCNand SCN-). 3. Formic acid is responsible for the sting in biting ants. By mass, formic acid is 26.10% C, 4.38% H, and 69.52% O. The molar mass of formic acid is 46.02 g/mol. Find the molecular formula of formic acid and draw its Lewis structure. 4. Consider the neutralization reaction: 5. Some of the important pollutants in the atmosphere are ozone (O3), sulfur dioxide, and sulfur trioxide. Write Lewis structures for these three molecules. Show all resonance structures where applicable. 6. Benzene (C6H6) consists of a six-membered ring of carbon atoms with one hydrogen bonded to each carbon. Write Lewis structures for benzene, including resonance structures. HCl + NaOH H2O + NaCl Write the reaction showing the Lewis structures of each of the reactants and products.