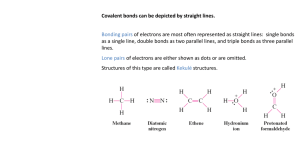
•'O-s-o
advertisement

Lewis Structures, Formal Charge, and Resonance 1. Draw Lewis structures for the following atoms showing all resonance structures. Use formal charges to determine which is the most appropriate arrangement of atoms or bonds. a. N2O Hoe" O b. S02 •'O-s-o O - S —O: 0.^ c. CO /Oe' 2. Draw Lewis structures for the following and assign formal charges as appropriate. Put formal charges in a different color ink. a. NO+ b. 022- «' .. c. H2C2O4 a name: -o - C - C - o - H C d. *expanded octet ..o-l-oI :0: •+2, Ir~ ' &' i' o = I -i? 3. Fill in the missing lone pairs. Assign formal charges to ALL atoms in this Lewis structure (use a different color ink.) Suggest a better resonance structure for this molecule. event (f M 4. Place the species below in order of shortest to longest nitrogen-oxygen bond. (Hint: Use bond order to determine this. Consider the best resonance structure and other possible contributing resonance structures and how they might affect the N-O bond order.) H2NOH 6 N2O n O M-0--H 0; = / It 0 r 1,0 NO+ -i NO2