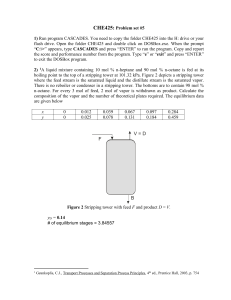
Chapter 2 - Testbank44
advertisement

Chapter 2 Solution 2.1 Basis for calculation: 100 kmol dry gas CO + 0.5O2 CO2 H2 + 0.5O2 H2O CH4 + 2O2 CO2 + 2H2O C2H6 + 3.5O2 2CO2 + 3H2O C6H6 + 7.5O2 6CO2 + 3H2O Reactions: REACTANTS O2 CO2 CO H2 CH4 C2H6 C6H6 N2 Nat. Gas 4 16 50 15 3 2 10 Totals 100 88.5 8 25 30 10.5 15 PRODUCTS CO2 4 16 15 6 12 H2O 50 30 9 6 10 53 95 If Air is N2:O2 = 79:21 N2 with combustion air = 88.5 x 79/21 = 332.9 kmol Excess O2 = 88.5 x 0.2 = 17.7 kmol Excess N2 =17.7 x 79/21 = 66.6 kmol Total = 417.2 kmol (i) Air for combustion = 417.2 + 88.5 = 505.7 kmol (ii) Flue Gas produced = 53 + 95 + 10 + 417.2 = 575.2 kmol (iii) Flue Gas analysis (dry basis): N2 CO2 O2 N2 409.5 kmol 53.0 kmol 17.7 kmol 480.2 kmol 85.3 mol % 11.0 mol % 3.7 mol % 100.0 mol % Solution 2.2 Use air as the tie substance – not absorbed. H2 O 0.05 % NH3 200 m3 s-1 760 mm Hg 20oC H2O 5 % NH3 © 2009 Elsevier Ltd. All rights reservedNH3 10 Partial volume of air = 200(1 - 0.05) = 190 m3 s-1 Let the volume of NH3 leaving the column be x, then: 0.05 x 100 190 x 0.05(190 + x) = 100x x 9.5 0.0950 m3 s-1 (100 0.05) (i) Flow rate of gas leaving column = 190 + 0.0950 = 190.1 m3 s-1 (ii) The volume of NH3 adsorbed = (200)(0.05) – 0.0950 = 9.905 m3 s-1 If 1 kmol of gas occupies 22.4 m3 at 760 mm Hg and 0oC, 273 9.905 Molar Flow = 0.412 kmol s-1 22.4 (273 20) Mass Flow = (0.412)(17) = 7.00 kg s-1 (iii) Let the water flow rate be W, then: 1 7.00 100 W 7.00 W = 700 – 7 = 693 kg s-1 Solution 2.3 OFF-GAS REFORMER 3 -1 2000 m h 2 bara 35oC H2 + CO2 + unreacted HC’s At low pressures vol% = mol% (i) Basis: 1 kmol of off-gas Component CH4 C2H6 C3H8 C4H10 mol% 77.5 9.5 8.5 4.5 MW 16 30 44 58 mass (kg) 12.40 2.85 3.74 2.61 21.60 So the average molecular mass = 21.6 kg kmol-1 *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 2 (ii) At STP, 1 kmol occupies 22.4 m3 5 2000 2 x 10 273 Flow rate of gas feed = 156.248 kmol h-1 5 22.4 1.013 x 10 (273 35) Mass flow rate = (156.248)(21.60) = 3375 kg h-1 (iii) Basis: 100 kmol of feed Reaction (1): CnH2n+2 + n(H2O) n(CO) + (2n + 1)H2 Component CH4 C2H6 C3H8 C4H10 n 1 2 3 4 Amount 77.5 9.5 8.5 4.5 If the conversion is 96%, then: CO 77.5 19.0 25.5 18.0 140.0 H2 232.5 47.5 59.5 40.5 380.0 H2 produced = (380.0)(0.96) = 364.8 kmol CO produced = (140.0)(0.96) = 134.4 kmol Reaction (2): CO + H2O CO2 + H2 If the conversion is 92%, then: H2 from CO = (134.4)(0.92) = 123.65 kmol Total H2 produced = 364.8 + 123.65 = 488.45 kmol/100 kmol feed If the gas feed flow rate = 156.25 kmol h-1, then 488.45 H2 produced = 156.25 763.20 kmol h-1 (763.2)(2) = 1526 kg h-1 100 Solution 2.4 ROH (Selectivity = 90 %) RCl ROR (Conversion = 97 %) Basis: 1000 kg RCl feed Relative molecular masses: CH2=CH-CH2Cl 76.5 CH2=CH-CH2OH 58.0 (CH2=CH-CH2)2O 98.0 *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 3 RCl feed = 1000 76.5 = 13.072 kmol RCl converted = (13.072)(0.97) ROH produced = (12.68)(0.9) ROR produced = 12.68 – 11.41 Mass of allyl-alcohol produced Mass of di-ally ether produced = 12.68 kmol = 11.41 kmol = 1.27 kmol = (11.41)(58.0) = 661.8 kg = (1.27)(98.0) = 124.5 kg Solution 2.5 Basis: 100 kmol nitrobenzene feed. The conversion of nitrobenzene is 96% and so 100(1 - 0.96) = 4 kmol are unreacted. The selectivity for aniline is 95% and so aniline produced = (96)(0.95) = 91.2 kmol Therefore, the balance is to cyclohexylamine = 96 – 91.2 = 4.8 kmol From the reaction equations: C6H5NO2 + 3H2 C6H5NH2 + 2H2O 1 mol of aniline requires 3 mol of H2 C6H5NO2 + 6H2 C6H11NH2 + 2H2O 1 mol of cyclohexylamine requires 6 mol of H2 Therefore, H2 required for the reactions = (91.2)(3) + (4.8)(6) = 302.4 kmol A purge must be taken from the recycle stream to maintain the inerts below 5%. At steady-state conditions: Flow of inerts in fresh H2 feed = Loss of inerts from purge stream Let the purge flow be x kmol and the purge composition be 5% inerts. Fresh H2 feed = H2 reacted + H2 lost in purge = 302.4 + (1 – 0.05)x Inerts in the feed at 0.005 mol fraction (0.5%) = (302.4 0.95 x) 0.005 1 0.005 = 1.520 + 4.774 x 10-3x Inerts lost in purge = 0.05x So, equating these quantities: 0.05x = 1.520 + 4.774 x 10-3x Therefore: x = 33.6 kmol The purge rate is 33.6 kmol per 100 kmol nitrobenzene feed. H2 lost in the purge = 33.6(1 – 0.05) = 31.92 kmol *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 4 Total H2 feed = 302.4 + 31.92 = 334.3 kmol Therefore: Total fresh hydrogen feed including inerts = 334.3 = 336.0 kmol 1 0.005 (iii) Composition at the reactor outlet: Stoichiometric H2 required for aniline = 300 kmol H2 feed to the reactor = (300)(3) = 900 kmol Fresh feed H2 = 334.3 and so recycle H2 = 900 – 334.3 = 565.7 kmol Inerts in Fresh Feed = (334.3)(0.005) = 1.6715 kmol 0.05 Inerts in Recycle (at 5%) = 565.7 = 29.77 kmol 1 0.05 Therefore, total inerts = 1.6715 + 29.77 = 31.445 kmol Aniline produced = 91.2 kmol Cyclohexylamine produced = 4.8 kmol If 302.4 kmol of H2 are reacted, then H2 leaving the reactor = 900 – 302.4 = 597.6 kmol H2O produced = (91.2)(2) + (4.8)(2) = 192 kmol Composition: kmol mol % Aniline 91.2 9.90 Cyclo-hexalymine 4.8 0.52 H2O 192 20.85 H2 597.6 64.89 Inerts 31.45 3.41 4 0.43 Nitrobenzene 921.05 100.00 Solution 2.6 AN Cyclo H2O H2 Inerts NB 950 10 1920 5640 300 40 H2 5640 Inerts 300 Pressure 20 psig = 1.38 barg Assumptions: H2 and inerts are not condensed within the condenser. Temp. = 270oC *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 5 Need to assume a condenser temperature. If cooling water is used as coolant then: Temp. of the gas at the condenser outlet = 50oC and return the cooling water at 30oC (20oC temp. difference). Antoine coefficients (from appendix C): Aniline 16.6748, 3857.52, -73.15 Nitrobenzene 16.1484, 4032.66, -71.81 H2O 18.3036, 3816.44, -46.13 Vapor pressures at 50oC: ln( P o ) 18.3036 H2O: 3816.44 323 46.13 Po = 91.78 mm Hg = 0.122 bar ln( P o ) 16.6748 Aniline: (From Steam Tables = 0.123 bar) 3857.52 323 73.15 Po = 3.44 mm Hg = 0.00459 bar Nitrobenzene: ln( P o ) 16.1484 4032.66 323 71.81 Po = 1.10 mm Hg = 0.00147 bar NB. The cyclo-hexalymine is ignored because it is present in such a small quantity. Mol fraction = partial pressure total pressure If the total pressure is 2.38 bara (20 psig): H2O = 0.122 = 0.0513 2.38 AN = 0.00459 = 0.0019 = 0.19 % 2.38 NB = 0.00147 = 0.00062 = 0.06 % 2.38 Total = 5.13 % 5.38 % Take H2 and the inerts as tie materials. Flow (H2 and inerts) = 5640 + 300 = 5940 kmol Mol fraction (H2 and inerts) = 100 – 5.38 = 94.62 % mol fraction other Flow of other components in vapor = mol fraction (H 2 inerts) H2O = flow (H 2 inerts) 5.13 x 5940 = 322.0 kmol 94.53 *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 6 AN = 0.19 x 5940 = 11.9 kmol 94.53 NB = 0.06 x 5940 = 3.8 kmol 94.53 Composition of the gas stream (recycle): kmol vol % H2 5640 89.84 Inerts 300 4.78 H2O 322.0 5.13 AN 11.9 0.19 NB 3.8 0.06 Cycl. Trace -- Total 6277.7 100.00 Composition of the liquid phase: Liquid Flow = Flow In – Flow in Gas Phase kmol kg vol % w/v % H2 0 -- -- -- Inerts 0 -- -- -- H2O 1920 - 322 1598 28764 61.9 23.7 AN 950 – 11.9 938.1 87243 36.3 71.8 NB 40 – 3.8 36.2 4453 1.4 3.7 10 990 0.4 0.8 121,450 100.0 100.0 Cycl. Total 2582.3 This calculation ignores the solubility of nitrobenzene in the condensed aniline. Note: H2O in the recycle gas would go through the reactor unreacted and would add to the tie H2O in the reactor outlet. But, as the recycle gas depends on the vapor pressure (i.e. the outlet temp.) it remains as calculated. The required flows of nitrobenzene and aniline are therefore: Inlet Stream: kmol vol % AN 950 10.34 Cycl. 10 0.11 2242 24.42 H2O 1920 + 322 *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 7 NB 40 0.44 H2 5640 61.42 Inerts 300 3.27 9182 100.00 Total An iterative calculation could be performed but it is not worthwhile. Solution 2.7 Basis: 100 kg feed AQUEOUS H20 AN NB Cycl 23.8 72.2 3.2 0.8 30oC 100.0 ORGANIC Minor components such as nitrobenzene and aniline will be neglected in the preliminary balance. Let the flow rate of aqueous stream be F kg per 100 kg of feed. Mass balances: IN Aqueous Organic xA (100 – F) Aniline 72.2 = 0.032F Nitrobenzene 3.2 = 3.2 Cyclohexylamine 0.8 = 0.0012F 0.01(100 – F) Water 23.8 = xW F 0.0515(100 – F) 1 301 3.2 300 301 Clearly the problem is over-specified, as we need to solve for 8 flow rates and we have 4 mass balances and 5 compositions specified. We can solve for F from the cyclohexylamine balance and then solve for xA and xW, but the resulting aqueous and organic stream flow rates will not necessarily sum to F and (100 – F): From cyclohexylamine balance: (0.01 – 0.0012) F = 1 – 0.8 F = 22.73 *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 8 Hence substituting in the other balances: IN Aqueous Organic Aniline 72.2 = 0.727 71.47 Nitrobenzene 3.2 = 0.0106 3.189 Cyclohexylamine 0.8 = 0.0273 0.773 Water 23.8 = 19.82 3.980 Total 100 20.59 79.41 So the aqueous phase does not sum to the previously determined value F = 22.73. If we iterate and substitute F = 20.6: IN Aqueous Organic Aniline 72.2 = 0.659 71.54 Nitrobenzene 3.2 = 0.0106 3.189 Cyclohexylamine 0.8 = 0.0247 0.794 X Water 23.8 = 19.71 4.089 Total 100 20.4 79.61 So the phase mass balances are now more or less correct, but the mass balance on cyclohexylamine does not close. It is more important that the mass balance closes properly than that the compositions exactly match those specified in the problem statement. In the case of cyclohexylamine, the composition in the organic phase is much larger than that in the aqueous phase, so if we exactly match the organic composition then the error in the aqueous composition will be large. If we exactly match the aqueous composition, the error in the organic composition will be small. We should therefore use the aqueous flow rate predicted, and solve for the organic flow rate by difference, giving: IN Aqueous Organic Aniline 72.2 = 0.659 71.54 Nitrobenzene 3.2 = 0.0106 3.189 Cyclohexylamine 0.8 = 0.0247 0.775 Water 23.8 = 19.71 4.089 Total 100 20.40 79.60 All phases and components now balance, and the error from the compositions defined in the problem statement is that the cyclohexylamine in the organic phase is 0.97% instead of the specified 1.0%. This error is acceptable for design purposes. [Note: At first glance this problem may just appear to be badly formulated, but it is in fact typical of many design problems, where the engineer may have redundant data and must resolve inconsistencies in the data.] *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 9 Solution 2.8 Calculation of the feed mol fractions: w/w kg/100 kg MW kmol/100 kg mol % H2O 2.4 3.05 18 0.169 14.1 AN 73.0 92.87 93 0.999 83.2 NB 3.2 4.07 123 0.0331 2.76 Total 78.6 100 1.201 100 Aniline in feed = 0.999 kmol h-1 With 99.9 % recovery, aniline on overheads = (0.999)(0.999) = 0.998 kmol h-1 Overhead composition will be near the azeotrope and so an aniline composition of 95 % is suggested. (NB: Would need an infinitely tall column to reach the azeotrope composition) Water composition in overheads = 100 – 95 = 5 mol % 5 So water carried over with the aniline = 0.998 = 0.0525 kmol h-1 95 Water leaving the column base = 0.169 – 0.0525 = 0.116 kmol h-1 Compositions: kmol h-1 mol % TOPS AN H2O NB 0.998 0.0525 Trace 1.505 95.0 5.0 Trace 100 BOTTOMS AN H2O NB 0.001 0.116 0.0331 0.150 0.66 77.73 22.1 100.5 Solution 2.9 Start by looking at the reaction stoichiometry: C7H8O2 + C3H6O2 = C10H14O4 *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 10 Stoichiometric equation balances, so no need to worry about additional components Molar weights: 124 74 198 i) 100 kg/day of guaifenesin = 100/198 = 0.505 kmol/day If plant yield is 87% then feed rate = 0.505/0.87 = 0.5805 kmol/day glycidine feed rate = 0.5805 74 = 42.96 kg/day So guaiacol feed rate = 0.5805 124 = 71.982 kg/day ii) Yield after reactor = feed rate 0.938 = 0.938 (100/0.87) = 107.82 kg/day So losses in evaporator = 107.82 – 100 = 7.82 kg/day iii) It is not necessary to read the patent to understand this part. The clue is that the catalyst is NaOH. The purpose of the evaporator must therefore be to evaporate the guaifenesin from the nonvolatile catalyst (which is actually neutralized with acid before the evaporator). The product that is lost is therefore the product that remains in the liquid phase in the evaporator. Possible ways to increase product recovery could include: Reducing the evaporator pressure Adding a second stage evaporator operated at lower pressure Stripping the reactor product using a suitable volatile compound (the patent says steam is no good, but glycidine could be investigated). Solution 2.10 It is not necessary to read the patent to answer this question. i) Start by writing a stoichiometric equation: C20H21ClN2 NMP-CDHBCP + C3H5ClO2 ECF = C22H23ClN2O2 Loratidine +X By difference, X has formula CH3Cl, i.e. is chloroform ii) Molar weights: C20H21ClN2 324.5 + C3H5ClO2 108.5 = C22H23ClN2O2 382.5 + CH3Cl 50.5 If conversion is 99.9%, then for 16.2g of feed NMP-CDHBCP, 16.2 0.999 = 16.184 g are converted So required feed of ECF = 16.184 (108.5/324.5) = 5.411 g Actual feed of ECF = 10.9g , so excess at end of reaction = 10.9 – 5.411 = 5.489 g *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 11 iii) If ECF is not recovered or recycled then 5.489/10.9 = 50.4% is lost to waste products (spent solvents). Since the selectivity is 100%, almost all of this lost material could in principle be recovered. iv) The feed of 16.2 g of NMP-CDHBCP gives a yield of 16.2 0.999 (382.5/324.5) = 19.076 g of product, so per kg of API we require 200 ml 1000/19.076 = 10.484 liters of each solvent at each step. Step solvent Reaction Quench Wash Recrystallization benzene water water isopropyl ether density (kg/m3) 879 1000 1000 724 mass (kg/200ml) 0.176 0.200 0.200 0.145 mass (kg/kg API) 9.216 10.484 10.484 7.590 In addition to the waste solvent, we also have chloroform produced by the reaction. Chloroform yield = 50.5 g per 382.5 g of API = 132.0 g/kg of API. The ECF waste is 5.489 g per 19.076 g product, i.e., 5.489 1000/19.076 = 287.7 g per kg product. The trituration step also uses petroleum ether, but the amount of this is much smaller than the amounts of solvents used. Allow 1 kg per kg product as an initial estimate. So total mass of waste produced is: Water Benzene Chloroform Ethyl chloroformate Petroleum ether Isopropyl ether Total 20.968 9.216 0.132 0.288 1.0 7.59 39.2 kg/kg API Note: this made no allowance for recovery yields on the washing and recrystallization steps, so the actual waste produced would be higher. v) 16.2 g of NMP-CDHBCP + 10.9 g of ECF give 19.076 g of product If 92% of the product is recovered after separation then overall yield = 0.92 19.076 = 17.550 g So to produce 10kg of API we require: Feed rate of NMP-CDHBCP = 16.2 (10/17.55) = 9.23 kg/batch Feed rate of ECF = 10.9 (10/17.55) = 6.21 kg/batch vi) The reactor should be operated no more than 2/3 full, to allow for swirling of the contents, vessel internals, etc., so available volume = 3800 2/3 = 2533 liters. Since the preparation involved decanting the benzene mixture into water, the reaction step must use only half of the available volume (so that the other equal volume of water can be added later), so available volume for reaction = 1267 liters The preparation method describes dissolving 16 g of NMP-CDHBCP in 175g of benzene (200ml), so the solution is roughly 10 wt%. We have no information on the *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 12 volume of mixing, so as a first approximation we can assume the mixed volume is 200ml. The lab recipe makes a 200ml batch, that ultimately yields 17.55 g of API. If 200ml gives 17.55 g, then: 1267 liters gives 17.55 (1267/0.2) = 111.2 kg per batch Reaction time is 18 hours. Estimated times for the other processing steps might be: Cooling & quench: 2 hours Decanting 1 hour Washing & evaporation 2 hours Trituration 1 hour Recrystallization 2 hours Total 8 hours If all the steps were carried out in the same vessel we would also need to allow time for cleaning and refilling at the end of the batch, say 2 more hours, giving a total batch time of 28 hours and production rate of 111.2/28 = 3.97 kg/hr. If only the reaction step is carried out in the reactor then we still need to allow some time for filling, emptying and cleaning, say 3 hours, giving a batch time of 21 hours and a maximum production rate of 111.2/21 = 5.3 kg/hr. vii) Advantages of carrying out all steps in same vessel: Less equipment to clean between batches Possibly higher yields during washing and decanting steps Disadvantages of carrying out all steps in same vessel: Could be impractical for trituration Could give poor separation during decanting, washing Difficult for ice-water quench unless the effect of pouring ice water into benzene mixture is the same as pouring benzene mixture into ice water (scaleup question). Would be difficult to get good recovery in final recrystallization step as material would coat vessel internals – easiest to send to a crystallizer. *You can buy complete chapters by: Www.TestbankU.com Contact Us: TestbankU@aol.com 13