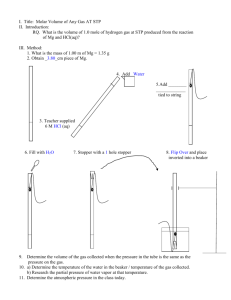
Universal and Specific Gas Constant
advertisement

Universal and Specific Gas Constant We know that the relation between pressure p, temperature T, volume V and mole n follows: ¤ The question now is that how can we determine this constant. In order to solve this problem, we should at first apprehend the well-known Avogadro’s Law. This law states that 1 mole of any gas occupies the same volume at the same pressure and temperature. Experiments prove that 1 kmol of every gas has the same volume of 22.4 m3 at standard condition, that is: standard pressure p0=101.325 kPa and standard temperature t0=0°C. Thus we have all the values of parameters: p0=101.325 kPa t0=0°C or T0=273.15K n0=1 kmol V0=22.4 m3 ¤ 101.325 10 22.4 273.15 /1000 8.31 And we call this universal gas constant and it is donated to R. Pay attention, as we have calculated, all the values we used are approximation. Therefore, the result 8.31 is also an approximation. It only provides you one possible approach to determine this universal gas constant. Usually R is given as: R=8.314 J/(mol K) In addition to universal gas constant, the specific gas constant is also needed: €•‚ƒ …‚ „ ƒ