Set5ans

CHE425: Problem set #5
1) Run program CASCADES. You need to copy the folder CHE425 into the H: drive or your flash drive. Open the folder CHE425 and double click on DOSBox.exe. When the prompt
“ C:\> ” appears, type CASCADES and press “ENTER” to run the program. Copy and report the score and performance number from the program. Type “ e
” or “ exit
” and press “ENTER” to exit the DOSBox program.
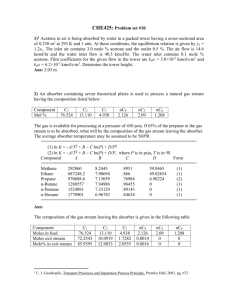
2) 1 A liquid mixture containing 10 mol % n-heptane and 90 mol % n-octane is fed at its boiling point to the top of a stripping tower at 101.32 kPa. Figure 2 depicts a stripping tower where the feed stream is the saturated liquid and the distillate stream is the saturated vapor.
There is no reboiler or condenser in a stripping tower. The bottoms are to contain 98 mol % n-octane. For every 3 mol of feed, 2 mol of vapor is withdrawn as product. Calculate the composition of the vapor and the number of theoretical plates required. The equilibrium data are given below x y
0
0
0.012
0.025
0.039
0.078
0.067
0.131
0.097
0.184
0.284
0.459
V = D
F
B
Figure 2 Stripping tower with feed F and product D = V. y
D
= 0.14
# of equilibrium stages = 3.84557
1 Geankoplis, C.J., Transport Processes and Separation Process Principles, 4 th ed., Prentice Hall, 2003, p. 754
3) 2 A liquid feed at the boiling point contains 3.3 mol % ethanol and 96.7 mol % water and enters the top tray of a stripping tower shown in Figure 3.
V, y
D
F x
F
L x
V y
S (Steam)
B, x
B
Figure 3 Stripping tower and direct steam injection .
Saturated steam is injected directly into liquid in the bottom of the tower. The overhead vapor which is withdrawn contains 99% of the alcohol in the feed. Assume equimolar overflow for this problem. Equilibrium data for mole fraction of alcohol are as follows at
101.32 kPa abs pressure.
x y
0
0
0.0080
0.0750
0.020
0.175
0.0296
0.250
0.033
0.270
(a) For an infinite number of theoretical steps, calculate the minimum moles of steam needed per mole of feed. (Be sure and plot the qline)
(b) Using twice the minimum moles of steam, calculate the number of theoretical steps needed, the composition of the overhead vapor, and the bottoms composition.
Ans. a) Minimum 0.121 mol steam/mol feed b) # of equilibrium stages = 4.73777
2 Geankoplis, C.J., Transport Processes and Separation Process Principles, 4 th ed., Prentice Hall, 2003, p. 754
4.
3 (a) For the cascade shown in Figure 4, calculate the composition of streams V
4
and L
1
.
Assume constant molar overflow, atmospheric pressure, saturated liquid and vapor feeds, and the vapor-liquid equilibrium data given below. x y
0
0
0.10
0.20
0.30
0.50
0.50
0.68
0.70
0.82
0.90
0.94
1.000
1.000
Total condenser
100 moles
70% alcohol
30% water
4
V
4 D
50 moles
L
R
4
V
4
3 3
2 2
1 1
L
1
100 moles
30% alcohol
70% water
L
1
100 moles
30% alcohol
70% water
(a)
(b)
Figure 4 Vapor-liquid equilibrium cascade.
(b) Given the feed compositions in cascade (a), how many stages would be required to produce a V
4
containing 85% alcohol?
(c) For the configuration in cascade (b), with D = 50 moles what are the composition of D and L
1
?
(d) For the configuration in cascade (b), how many stages are required to produce a D of
50% alcohol?
Ans. a) x5 = 0.700456
3 J. D. Seader and E. J. Henley, Separation Process Principles, Wiley, 1998
b) A vapor stream containing 85% alcohol cannot be obtained (infinite number of stages required). c) xD( between 0.3 and 0.95) = .445 y0 = 0.300531, x1 = 0.156062 d) The operating line intersects equilibrium curve
infinite number of stage.
5.
Determine the overall efficiency of a distillation column used to separate methanol from water. The average temperature and pressure of the column are 80 o
C and 1 atm, respectively.
The feed is saturated liquid with mole fraction of methanol equal to 0.40 corresponding to equilibrium vapor mole fraction of 0.718.
Ans. 0.4986
6.
Determine the plate efficiency of a distillation column used to separate methanol from water. The plate is at 80 o
C and 1 atm. The cross-sectional area of the column is 0.50 m
2
. The vapor volumetric flow rate is 0.95 m
L
(kg/m
3
)
v
(kg/m
3
)
L
3
/s. The following data are provided
(kg/m
s)
v
(kg/m
s) h w
(m)
806 0.826 0.336×10
-3
10.6×10
-6 50×10 -3
A h
(m
2
0.038
)
Atomic volume of m 3 /kmol
C
0.0148
Ans. The efficiency of the plate is 67%.
H
0.0037
O
0.0074
7.
A liquid mixture of benzene-toluene is to be distilled in a fractionating tower at 760 mmHg. The feed is saturated liquid with a flow rate of 100 kmol/h, containing 50 mol % benzene and 50 mol % toluene, and enters at 327.6 K. We want to obtain a distillate containing 95 mol % benzene and 5 mol % toluene and a bottoms containing 2 mol % benzene and 98 mol % toluene. The reflux ratio is 2.0. The average heat capacity of the feed is 159 kJ/kmol and the average latent heat 32,099 kJ/kmol. Use enthalpy balances to determine the temperatures, flow rates, and composition of the streams entering and leaving the first two stages of the column (after the condenser).
Physical property data are given in the following Table 1 1 .
Component T b
( o
C) C p
(kJ/kmol
K) C py
(kJ/kmol
K)
(kJ/kmol)
Benzene (A)
Toluene (B)
80.1
110.6
138.2
167.5
96.3
138.2
30,820
33,330
Vapor pressure: P sat
(mmHg), T ( o
C) log
10
P
A sat log
10
P
B sat
= 6.90565
1211.033/( T + 220.79)
= 6.95334
1343.943/( T + 219.377)
You need to provide the following information
Stage 1: T
1
( o
C) = __82.57__ Stage 2: T
2
( o
C) = __84.65__
L
0
(kmol/h) L
1
(kmol/h) L
2
(kmol/h)
103.2258 102.07 99.73
V
1
(kmol/h) V
2
(kmol/h) V
3
(kmol/h)
154.8387 153.69 151.34
Note: x and y are the mole fraction of benzene. x y
0
0.95
1
0.95 x
1
0.88104 y
2
0.90420 x
2
0.78754 y
3
0.84294
1 Geankoplis, C.J., Transport Processes and Separation Process Principles, 4 th edition, Prentice Hall, 2003, p.
736