P-v-T Relations for Vapors (Gases)
advertisement
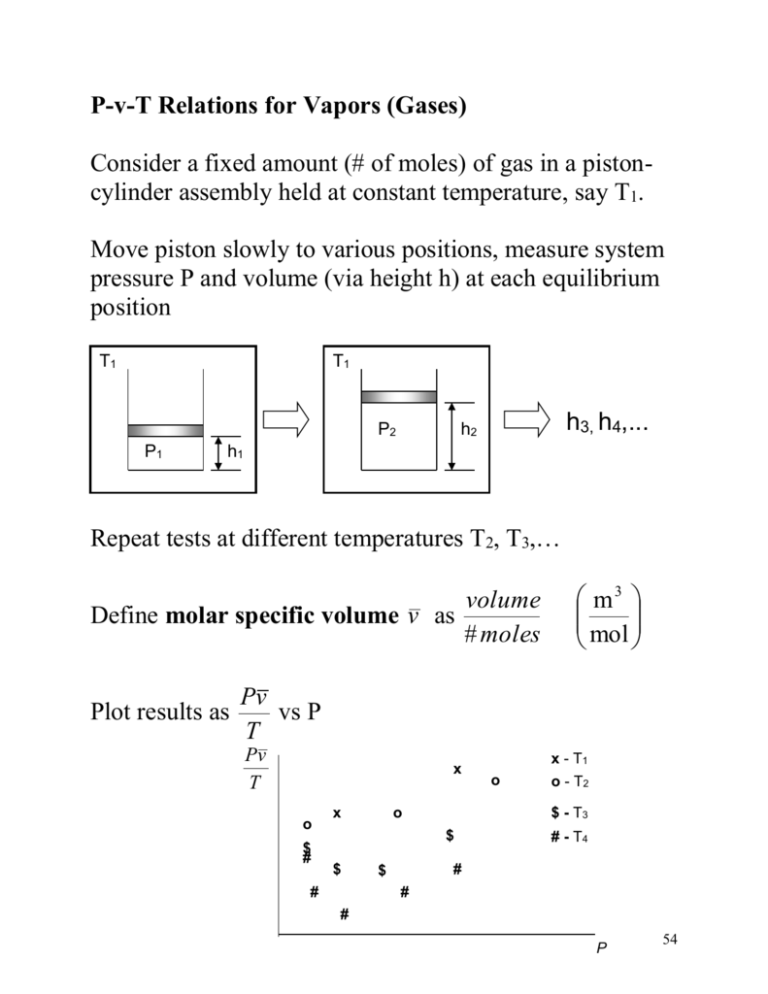
P-v-T Relations for Vapors (Gases) Consider a fixed amount (# of moles) of gas in a pistoncylinder assembly held at constant temperature, say T1. Move piston slowly to various positions, measure system pressure P and volume (via height h) at each equilibrium position T1 T1 P2 P1 h3, h4,... h2 h1 Repeat tests at different temperatures T2, T3,… volume Define molar specific volume v as # moles Plot results as m3 mol Pv vs P T Pv T x o $ # x o $ - T3 $ $ # o x - T1 o - T2 # - T4 # $ # # P 54 Since Pv is a finite number, as P 0, v As v (volume/#moles) approaches infinity continuum assumption fails have to extrapolate experimental data to P= 0 x Pv T x x x x x x x x x T1 T2 T3 T4 x x x P Extrapolated data It is found that when the data is extrapolated to P= 0 you get a limiting value for Pv / T : N m3 2 m kmol Nm Pv lim R units : o P 0 T K kmol oK The same value for R is found for all gases, hence it is referred to as the Universal Gas Constant R 8314 J kJ 8 . 314 kmol oK kmol oK 55 The ratio of the molar and mass specific volume defines the Molar mass, : v vol mass mass v mol vol mol v v Pv Pv lim lim R P 0 T P 0 T Pv R lim P 0 T Define the gas constant as, R R / The gas constant for air is Rair J kmol J 8314 286 . 7 kmol oK 29kg kg oK R We define the compressibility factor Z as Z Pv P ( v) Pv R T ( R )T RT 56 Re-plotting data as Z vs P (as P 0, Z 1 x x x x x x x x x x Pv R , Z 1) T T1 T2 T3 T4 x x x P Repeat experiments using different gases and get the same shape curves. If we plot Z versus the reduced pressure PR (=P/Pc) for different reduced temperatures TR (=T/Tc) you get a universal set of curves known as the generalized compressibility chart Molar mass and critical properties are given in Table A-1, for example: Tc(air)= 133K Pc(air)= 37.7 bar Tc(water)= 647.3K Pc(water)= 220.9 bar 57 For a given gas with a gas constant R= R / , knowing the pressure and temperature you can use the above graph to v get the specific volume v Z 1 (within 5%) for low reduced pressure (PR < 0.2) independent of temperature, e.g. Pair < 7.6 bar Z 1 (within 5%) for high reduced temperatures (TR > 2) independent of pressure, e.g. for air Tair > 266K (-7C) 58 In most engineering applications we deal with gases at conditions that are within these limits, so Z1 A gas for which Z can be assumed equal to one is called a perfect gas, or an ideal gas Pv Pv For an ideal gas Z 1 R T RT Pv R T and since v V/M so Pv RT P RT and v V/n PV nR T and n # moles PV MRT Microscopic Point of View Molecules have KE associated with motion and PE associated with mutual attraction/repulsion. An ideal gas is one where the PE is negligible, e.g., billiard ball model. At low pressure molecules are far apart so negligible PE At high temp KE >> PE 59











