Set10ans
advertisement
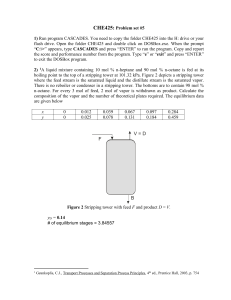
CHE425: Problem set #10 1)1 Acetone in air is being absorbed by water in a packed tower having a cross-sectional area of 0.250 m2 at 293 K and 1 atm. At these conditions, the equilibrium relation is given by ye = 1.2xe. The inlet air contains 3.0 mole % acetone and the outlet 0.5 %. The air flow is 14.6 kmol/hr and the water inlet flow is 40.5 kmol/hr. The water inlet contains 0.1 mole % acetone. Film coefficients for the given flow in the tower are kya = 3.8×10-2 kmol/s∙m3 and kxa = 6.2×10-2 kmol/s∙m3. Determine the tower height. Ans: 2.03 m 2) An absorber containing seven theoretical plates is used to process a natural gas stream having the composition listed below: Component Mol % C1 76.524 C2 13.110 C3 4.938 nC4 2.126 nC5 2.09 nC6 1.208 The gas is available for processing at a pressure of 450 psia. If 65% of the propane in the gas stream is to be absorbed, what will be the composition of the gas stream leaving the absorber. The average absorber temperature may be assumed to be 560oR. ============================================================= (1) ln K = A/T2 + B C ln(P) + D/P2 (2) ln K = A/T2 + B C ln(P) + D/P, where P is in psia, T is in oR Compound A B C D Form ============================================================= Methane 292860 8.2445 .8951 59.8465 (1) Ethane 687248.2 7.90694 .866 49.02654 (1) Propane 970688.6 7.15059 .76984 6.90224 (2) n-Butane 1280557 7.94986 .96455 0 (1) n-Pentane 1524891 7.33129 .89143 0 (1) n-Hexane 1778901 6.96783 .84634 0 (1) ============================================================= Ans: The composition of the gas stream leaving the absorber is given in the following table Component Moles in feed Moles exit stream Mole% in exit stream 1 C1 76.524 72.2543 85.9395 C2 13.110 10.0919 12.0033 C3 4.938 1.7282 2.0555 nC4 2.126 0.0014 0.0016 nC5 2.09 0 0 nC6 1.208 0 0 C. J. Geankoplis, Transport Processes and Separation Process Principle, Prentice Hall, 2003, pg. 672 3. 2Solute A is to be removed from an inert gas B in a multi-stage countercurrent absorption tower. The gas enters the tower at a rate of 200 kmol/h and contains 25 mol% A. The solvent enters the tower at a rate of 800 kmol/h and is initially free of solute. Determine (a) the concentration of the exiting gas stream and (b) the number of stages, if the exiting liquid stream contains 5.0 mol% A. The equilibrium relationship is yA = 4.0xA. Assume that the carrier gas is insoluble in the solvent and the solvent is nonvolatile. Ans: Yt = 0.053, yt = 0.050 Number of equilibrium stages = 3.62657 4. 3Solute A is to be stripped from a liquid stream by contacting the liquid with a pure gas. The liquid enters the stripping tower at an A-free rate of 150 kmol/h and contains 30 mol% A. The gas enters the column at a rate of 500 kmol/h. Determine the number of stages required to reduce the concentration of A in the exiting liquid stream to 1.0 mol%. The distribution of A in the gas and liquid phase is expressed as yA = 0.4xA. Yt = 0.126, yt = 0.112 Number of equilibrium stages = 11.6379 2 3 Hines, A. L. and Maddox R. N., Mass Transfer: Fundamentals and Applications, Prentice Hall, 1985, pg. 280 Hines, A. L. and Maddox R. N., Mass Transfer: Fundamentals and Applications, Prentice Hall, 1985, pg. 280 5. Using the enthalpy-composition diagram for the methanol-water system (Figure 1), calculate the number of stages required and the feed plate location to produce 98 wt% methanol liquid product and 5 wt% methanol bottoms product from a feed of 45 wt% methanol entering the column at 50oC. The column has a total condenser and a partial reboiler, and operates at 1.5 times minimum reflux. What is the reboiler duty in Btu/hr if the feed rate is 5,000 lb/hr? What is the condenser duty? Ans: QD = 2.37×106 Btu/hr QR = 2.77×106 Btu/hr From the chart, need 8 eq. trays + 1 reboiler. 6. Solve the following set of linear equations1 using the Thomas algorithm. 0 2 3 0 1 2 1 0 0 4 1 1 2 1 0 0 y1 4 y 2 2 = y3 9 y4 2 List the coefficient matrix after the elimination step. The matrix should have the following form 1 0 0 0 p1 0 1 0 0 p2 1 0 0 y1 q1 0 y2 q2 p3 y3 q3 1 y4 q4 Ans: 1 0 0 0 0 y1 2 1 3.5 0 y2 1.14 0 1 0.143 y3 31 0 0 1 y4 4 2 0 y4 = 4 1 =4 y3 = (3 c3 y4)/3 = [31 1(4)]/0.143 =3 y2 = (2 c2 y3)/2 = [1.14 (1)(3)]/3.5 =2 y1 = (1 c1 y2)/1 = [2 (3)(2)]/2 =1 Riggs, J. B. An Introduction to Numerical Methods for Chemical Engineers, Texas Tech University Press, 1994, p. 39