File
advertisement

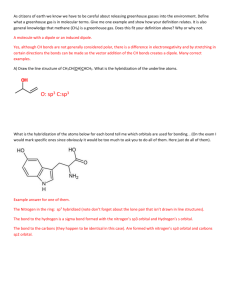
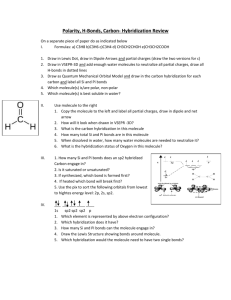
ATOMIC STRUCTURE ONE WORD 1. Who discovered cathode rays? a) Ruther ford b) Goldstein c) Dalton d) Thomson 2. ……….. was discovered by Anderson. a) nucleus b) electron c) neutron d) positron 3. Dual character of electrons was explained by ……… a)Bhor b) Heisenberg c) de broglie d) pauli 4. de Broglie equation is ............ a) hv=mc2 b) (hc/lambda)=mc2 c) lambda=h/mc d) all of these 5. The circumference of the circular orbit of an electron must be an integral multiple of the ……….wave. a) wavelength, IR b) wavelength, UV c) wavelength, de Broglie d) wavenumber, Newton. 6. The size of the s orbital depends upon the value of ……… quantum number. a) spin b) principal c) azimuthal d)spin 7. The mass of electron is …… Kg. a) 9.1 *10 (-31) b) 9.1 *10(-340 c) 9.1 *10(-33) d) 9.1 * 10(-13). 8. Antineutrino was discovered by……….. a) Planck b) Fermi c)de Broglie d)Bhor 9. A de Broglie wavelength of 1 angstrom will be obtained if the applied voltage is approximately……….. a) 150v b) 1.5v c) 15v d) 15mv 10. Atomic number was proposed by…….. a) Planck b) Maxwell c) Mosley d) Mendelef 11. Who discovered meson? a) Yuwakawa b) Yukawa c) Young d) Euler 12. The antiproton was discovered by …….. a) Chadwick b)Fermi c) Segre d) Goldstein 13. Cork and his group discovered …… a) antiproton b) antineutron c) antielectron d) antineutrino 14. Davisson and Germer performed the ……… experiment. a) electron acceleration b) proton acceleration c) electron diffraction d) proton diffraction 15. The e/m ratio of the electron was determined by…….. a) Milliken b) Mulliken c) Bhor d) Thomson 16. The black body radiation is due to ……..nature of the electron. a) particle b) wave c) both a and b d) neither a nor b 17.The photoelectric effect is due to……….. nature of electron a) particle b) wave c) both a and b d) neither a nor b 18. The Davisson and germer experiment confirms the ……….nature of electron. a) particle b) wave c) both a and b d) neither a nor b 19.Diffraction and interference are characteristic of………….. a) particle b) wave c) both a and b d) neither a nor b 20. The electromagnetic theory was proposed by…….. a) Bhor b) Maxwell c) Plucker d) Milliken 21. Elliptical orbits are introduced by ………. a) Sony b) Sommerfield c) Plucker d) Milliken 22. Energy of the single electron in hydrogen if it were in the second orbit is …………..Kj per mole. a) -1312 b) -328 c) -656 d) 0 23.The ionization energy of hydrogen I the ground state is -1312kj per mole. If its energy in the second orbit is -329kj per mole , the energy that would be released from the second orbit would be ……. Kj per mole. a) -1312 b) -328 c) -656 d) 984 24.The ionization energy of hydrogen in the ground state is ……….. a) -1312 b) -328 c) -656 d) 0 25. de Broglie waves have found application in ……… microscopy a) optical b) electron c) neutron d) positron 26. …….. proposed the uncertainity principle a) Hund b) Heisenberg c) Pauli d) Mosley 27. The circular orbit of the electron should be a integral multiple of the wavelength of ………….. wave a) de Broglie b) electric c) light d) sound 28. P=h/lambda is known as ……… equation . a) de Broglie b) Einstein c) Einstein- de Broglie d) Einstein-Mulliken 29. The energy of a electron at infinity is assumed to be ……….. a) infinity b) positive integer c) zero d) negative 30. When an electron is at infinite distance from the nucleus, it is said to be at ………. Energy. a) zero b) infinite c) positive d) negative 31. The energy required to shift an electron from the first orbit to an infinite orbit is known as ……….. energy. a) excitation b) transition c) ionization d) polarization 32. Ethylene is an example for a ………. Shape molecule. a) linear b) octahedral c) triangular planar d) tetrahedral 33. The distribution of electrons among various molecular orbitals is called ……… configuration. a) atomic b) electronic c) MO d) orbital 34. The stability of a molecular or an ion can be determined from ……. Order. a) order b) molecular c) ionic d) bond 35. Half of the difference between the number of electrons in the bonding and antibonding orbitals is ………. Order. a) band b) broad c) bond d) beam 36. The filling up of atomic orbitals in the increasing order of energy levels is that of ……… a) Aufbau b) Hund c) Pauli d) Bhor 37. ………… is the space around the nucleus having the maximum probability of finding an electron a) Orbit b) orbital c) shell d) Niche 38. Given that the kinetic energy is the same, which of the following will have the maximum de Broglie wavelength? a) Alpha particle b) proton c) beta particle d) neutron 39. The value of Bhor’s radius for hydrogen is ……….. cm. a) 0.52* 10(-8) b) 0.52*10(-10) c) 0.52*10(-6) d) 0.52 * 10(-12) 40. The energy of the single electron in the grond state of hydrogen atom is …………. J. a) 1.938*10(-15) b) 1.938 *10(-16) c) 1.938 *10(-17) d) 1.938*10(-18) 41. The hybridization of sf6 molecule is …….. a) sp3d3 b) sp3 c) sp3d2 d) sp2d 42. The hybridization in [ PtCl4]2- molecule is …….. a) sp2d b) sp3 c) sp3d2 d) sp3d 43. The hybridization in PF5 molecule is ……… a) sp3d3 b) sp3 c) sp3d2 c) sp3d 44. the hybridization in IF7 molecule is ……… a) sp3d3 b) sp3 c) sp3d2 d) sp3d 45. The hybridization in NO2- ion is ……… a) sp3d b) sp3 c) sp3d2 d) sp2 46. The hybridization in NO3- ion is ……. a) sp3d2 b) sp2 c) sp2d3 sp3d 47. The hybridization in SO4- ion is ………… a) sp3 b) sp2 c) sp3d2 d) sp3d 48. The hybridization in CO3- ion is ………. a) sp3d3 b) sp3 c) sp2 d) sp3d 49. The hybridization in NH4- ion is …………… a) sp3d3 b) sp3d c) sp2 d) sp3 50. The hybridization in ClO4- ion is ……….. a) sp3d3 b) sp3d c) sp2 d)sp3 51. The hybridization in BH3 molecule is ……… a) sp3d3 b) sp3 c) sp2 d) sp2 52. The hybridization in BeF2 molecule is …………. a) sp3d3 b) sp3 c) sp2 d) sp 53. The hybridization in acetylene molecule is …………. a) sp3d3 b) sp3 c) sp2 d) sp3d 54. The hybridization in ClO2- ion is ……… a) sp3d3 b) sp3 c) sp2 d) sp3d 55. The hybridization in Clo3- ion is ………. a) sp3d3 b) sp3 c) sp2 d) sp2d 56. The hybridization in Co2 molecule is ……. a) sp3d3 b) sp3 c) sp d) sp3d 57.The hybridization in BrF5 molecule is ……… a) sp3d3 b) sp3 c0 sp3d2 d) sp3d 58. The hybridization in XeOF4 molecule is ….. a) sp3d2 b) sp3 c) sp d) sp3d 59. The hybridization in carbon tetrachloride molecule is ………. a) sp3d3 b) sp c) sp3 d) sp3d 60.The hybridization in hydronium ion (H3O)+ is ……… a) sp3d3 b) sp3 c) sp d) sp3d 61. The hybridization in nitrogen triflouride molecule is ……… a) sp3d3 b) sp c) sp3 d) sp3d 62. The hybridization in CIF3 molecule is ……….. a) sp3d3 b) sp3 c) sp d) sp3d 63. The hybridization in water molecule is ………. a) sp3d3 b) sp3d3 c) sp3 d) sp3d 64. The hybridization in mercuric chloride molecule is ……… a) sp3d3 b) sp3 c) sp d) sp3d 65. The hybridization in ethylene molecule is ………. a) sp3d3 b) sp3 c) sp2 d) sp3d 66. The hybridization in BeH2 molecule is ……….. a) sp3d3 b) sp3 c) sp d) sp3d 67.The head-on overlap of atomic orbital results in ……… bond. a) alpha b) beta c) sigma d) pi 68. If all the electrons in a molecule are paired then the molecule is……… a) paramagnetic b) diamagnetic c) sometimes paramagnetic and mostly diamagnetic d) sometimes diamagnetic and mostly paramagnetic 69. If all the electrons in a molecule is unpaired then the molecule is …………….. a) paramagnetic b) diamagnetic c) sometimes paramagnetic and mostly diamagnetic d) sometimes diamagnetic and mostly paramagnetic 70. Hund and Mulliken proposed the ……….. theory. a) magnetic spin b) electronic spin c) coordination d) molecular orbital 71. The number of molecular orbitals formed is ……… the number of atomic orbitals. a) not related to b) equal to c) more than d) lesser than 72. The molecular orbital having lower energy is known as ……. a) bonding b) antibonding c) probonding d) parabonding 73. The molecular having higher energy is known as ………. a) antibonding b) bonding c) probonding d) prebonding 74. The bonding molecular orbitals are not represented by……….. a) alpha b)beta c) sigma d) pi 75. The antibonding molecular orbitals are not represented by……. a) alpha* b) beta* c) sigma* d) pi* 76. The shapes of molecular orbitals depend upon ……… of combining atomic orbitals. a) density b) volume c) shape d) size 77. In caseof heteronuclear molecules, atomic orbitals of ……. energy combine to form the molecular orbitals. a) different b) near-equal c) zero d) infinite. 78. In the case of homonuclear molecules, the orbitals involved in hybridization must have……. Energy. a) different b) equal c) zero d) infinite. 79. The lateral overlap of atomic orbital results in …… bond. a) alpha b) beta c) sigma d) pi. 80. Bond length is ........ related to bond order. a) directly b) inversely c) not d) sometimes directly and sometimes indirectly. 81. Bond order is given by the expression …… a) (Nb-Na)/2 b) (Nb-Na)/3 c) (Nb-Na) d) 2(Nb-Na). 82. Bond order can be ........ a) 100 b) a fraction c) infinity d) zero. 83. The bond order in nitrogen molecule is …… the bond order in oxygen molecule. a) longer than b) same as c) shorter than d) much longer than. 84. For s orbitals, when l=0 the value of m is …. a) 0 b) 1 c) 10 d) 100. 85. An important feature of the 2s orbital is, it has a node where the possibility of finding the electron is ….. a) minimum b) maximum c) infinite d) zero. 86. Orbitals having same energy are known as …… set of orbitals. a) degenerate b) nondegenerate c) generate d) nongenerate. 87. There are ….. lobes in any d orbital . a) 1 b) 3 c) 4 d) 2. 88. In the filling up of molecular orbitals, ……. Will not take place until the degenerate orbitals have one electron each. a) unpairing b) pairing c) sharing d) unsharing. 89. Molecular orbitals are filled in the ….. order of their ….. a) decreasing, energy b) increasing, energy c) decreasing, volume d) increasing, energy. 90. According to Pauli’s exclusion principle, the two electrons occupying a molecular orbital should have ….. spin a) same b) no c) opposite d) sometimes same sometimes opposite. 91. The two hydrogen atoms in a hydrogen molecule are connected by….. bond(s). a) a single sigma b) a single pi c) single sigma and single pi d) two pi. 92. Since no unpaired electrons are present, the hydrogen molecule is…… a) paramagnetic b) diamagnetic c) sometimes paramagnetic mostly diamagnetic d) sometimes diamagnetic mostly paramagnetic. 93. The bond order of He2 comes out to be zero. Therefore He2 molecule …… exixts. a) can exist b) can’t exist c) can exist at times d) none 94. The hydrogen bridge network in water is responsible for its …. Nature. a) boiling b) liquid c) tasteless d) colourless. 95. In Which of the following molecules is hydrogen bonding absent? a) water b) hydrogen fluoride c) carbon tetrachloride d) ethyl alcohol 96. Intramolecular hydrogen bonding is not characteristic of …. a) o-nitrophenol b) salicylic acid c) salicylaldehyde d) carbolic acid. 97. The stability of a molecule is directly proportional to … a) bond length b) bond order c) color d) density. 98. If Nb >Na the molecule is ….. a) moderately stable b) stable c) unstable d)very unstable. 99. If Na>Nb the molecule is …. a) moderately stable b) stable c) unstable d) very stable. 100. If Na=Nb the molecule is …. a) moderately stable b) stable c) unstable d) very stable.