Exam #2 – Electrons, Compounds, and Structure
advertisement

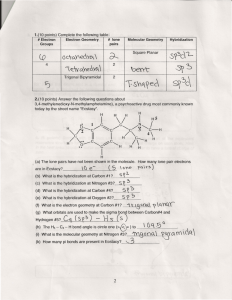
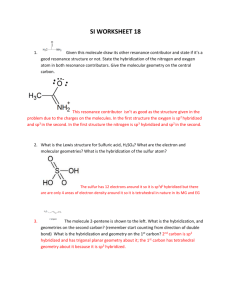
Chemistry 161 Exam 2, June 2nd, 2010 Name: ____________________ Show all work for full credit Exam #2 – Structure and Stoichiometry SHOW ALL OF YOUR WORK TO RECEIVE FULL CREDIT Make a note of the point value of each question, and allocate your time accordingly. Carefully read each question. Where appropriate, work must be shown to receive proper credit. Take your time, make sure to check your work for mistakes when you are finished. Partial credit will be awarded based on work shown. If you get stuck, move on to another problem. Use a pencil so that you can erase any mistakes. Page Points 2 40 3 22 4 38 TOTAL 100 Score 1/4 Chemistry 161 Exam 2, June 2nd, 2010 1. (40 points) Chemical Formula Name: ____________________ Show all work for full credit Draw the Lewis Dot Structure (Include ALL lone pair electrons and RESONANCE structures) Is the compound polar? If yes, draw the net dipole Molecular Geometry And Electron Geometry Shape: PO23Electron Geometry: Shape: XeCl4 Electron geometry: Shape: SeO3 Electron Geometry: Shape: IBr3 Electron Geometry: 2/4 What are the Bond Angles? (X-A-X) What is the Hybridization state of the central atom? Chemistry 161 Name: ____________________ nd Exam 2, June 2 , 2010 Show all work for full credit 2. a. (10) The bonding in the diatomic molecule C2 can be explained using molecular orbital theory. Draw an energy diagram that represents the molecular orbitals created when the C2 molecule is formed, and fill in these orbitals with the appropriate number of electrons. (label your orbitals for full credit) b. (2) What is the bond order of this molecule? c. (2) What can you say about the magnetic properties of the C2 molecule? 3. (8) Answer questions about the following molecule: .. .. .. a .. a. (2) How many sigma bonds does this molecule have? (Be careful!) _______ b. (3) How many pi bonds does this molecule have? Circle each of these pi bonds for full credit. _______ c. (1.5) What is the hybridization state of the carbon labeled with an “a”? _______ d. (1.5) What is the formal charge of the terminal nitrogen (right one)? 3/4 _______ Chemistry 161 Name: ____________________ nd Exam 2, June 2 , 2010 Show all work for full credit 4. (11) Fill in any blanks in the following table (for instance, give the formula if the name is given): Compound Formula Covalent or Ionic? Name of compound CO SrCl2 CrO Ammonium sulfite Cu2O PO4 Vanadium (II) Nitride 5. (27) 382.33 grams of ammonia react with 751.35 grams of oxygen gas in the following balanced reaction: 2NH3 (g) + 3O2 (g) N2O3 (g) + 3H2O (g) a) (7 points) Determine the limiting reagent b) (7 points) Calculate the theoretical yield of dinitrogen trioxide produced (in grams). c) (8 points) Calculate the moles of unreacted reactant. d) (5 points) My lab typically has a 47.3% yield for the above reaction. What will my actual yield be for this reaction given the reactant quantities indicated above (in grams). 4/4