Syllabus: ORGANIC CHEMISTRY I & II LABORATORY
advertisement

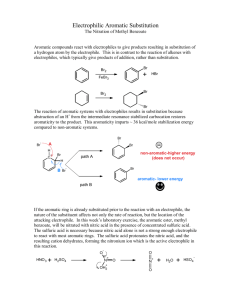
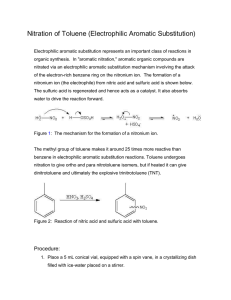
WEEK 5: NITRATION REACTION PURPOSE: This experiment will introduce the student to one of the important electrophilic aromatic substitution reactions. The experiment uses concentrated nitric acid and concentrated sulfuric acid so the student will need to use great care in handling these reagents. IMPORTANT REACTIONS: NO2+ + 2 HSO4- + H3O+ HNO3 + 2 H2SO4 Nitronium ion CO2CH3 CO2CH3 HNO3 / H2SO4 NO2 Methyl benzoate (M. Mass 136.16, bp 199.6oC) Methyl 3-nitrobenzoate (M. Mass 181.15, mp 78oC) BACKGROUND INFORMATION: This experiment and the one next week are examples of electrophilic aromatic substitution reactions. Aromatic systems such as the benzene ring have enhanced resistance toward reactions due to the stability that resonance gives to the aromatic ring. Addition reactions are highly unlikely as they would destroy the conjugated aromatic system. Substitutions can occur and the best reagent to use should have electrophilic properties as the aromatic ring is rich in electrons. The class of reactions known as electrophilic aromatic substitution reactions will be studied extensively in the lecture part of the course. These two experiments will show how they can be performed in the laboratory. There are five reactions that are normally presented when discussing electrophilic aromatic substitution reactions. They include: 1. nitration using concentrated nitric acid and concentrated sulfuric acid to generate the nitronium ion electrophile (which will be done in this experiment). 2. halogenation using a halogen and a Lewis acid to generate the halonium ion elctrophile. 3. sulfonation using sulfur trioxide as the electrophile. This is the only neutral electrophile in this series of reactions and this reaction is reversible. 4. Friedel Crafts alkylation using some source of a carbenium ion as the electrophile. This reaction will be done next week and will use an alcohol and concentrated sulfuric acid to generate the carbenium ion. 5. Friedel Crafts acylation using an acyl halide and a Lewis acid to generate the acylium ion electrophile. In the experiment that will be done today, a substituted aromatic ring is used which will lead to meta nitration of the ring. The substituent chosen will moderate the reaction to avoid di- and trisubstitution of the ring which could lead to explosive products. Trinitrobenzene is explosive. It is best not to heat the reaction too high or too long to avoid further undesired substitution. Also note that this reaction often shows a delayed start. It is best to be patient. If the mixture is heated highly or longer than directed, an uncontrolled reaction may ensue. And, if the reaction is quickly quenched in an ice bath, it may be permanently stopped. If brown fumes start to form, the reaction may be proceeding too quickly and should be moderated. Concentrated nitric acid and concentrated sulfuric acid pose a hazard when being handled. Gloves should be worn and care must be used. If any of the acid mixture gets on the skin, it must be immediately washed with large amounts of cold water. Immediately use a water wash if you suspect that acid may be on your skin. Nitric acid destroys proteins and if some of it (either as liquid or vapors) gets on the skin, the skin cells will die over a few hours to days leaving an orange patch on the surface. This generally wears off in a few days. This reaction usually goes well if care is exercised. The final product is a crystalline solid with a low melting point. EXPERIMENTAL PROCEDURE: Carefully combine concentrated sulfuric acid (0.4 mL) and concentrated nitric acid (0.4 mL) in a micro-scale kit reaction tube and place it in an ice bath. To a second tube, add methyl benzoate (0.6 g, 4.4 mmoles) and concentrated sulfuric acid (1.2 mL), flick the flask to mix the components of this viscous mixture, then cool this tube the ice bath as well. Using a glass Pasteur pipette, carefully add the sulfuric-nitric acid mixture dropwise to the methyl benzoate solution, using a glass stirring rod to mix the reaction. On complete addition of the acid mixture, remove the reaction from the ice bath, allow it to warm up to room temperature, and let is stand for 15 mins. After this time, in order to crystallize out the product, pour the mixture into a small beaker that contains approximately 5g of ice. Filter the resulting solid using a Hirsch funnel and wash out the reaction tube with ice-cold water. When all the product has been transferred to the filtration funnel, wash the product with ice-cold methanol (0.5 mL). Record the mass and melting point of this crude product and calculate the percent yield. Recrystallize the product using an equal mass of methanol and re-record the mass, melting point and percent yield of the pure product. IMPORTANT INFORMATION ABOUT THE REPORT: The report for this experiment will follow the usual format. Be sure the percent yield calculation is carefully done. Also, record the melting point range of the final product and compare that melting point to the reported melting point of methyl 3-nitrobenzoate. Using these data, discuss the relative success on the experiment and discuss whether there is any evidence polysubstitution occurred. END OF EXPERIMENT. © 2007 STEPHEN ANDERSON AND ROBERT SHINE