Nitration of Methyl Benzoate
advertisement
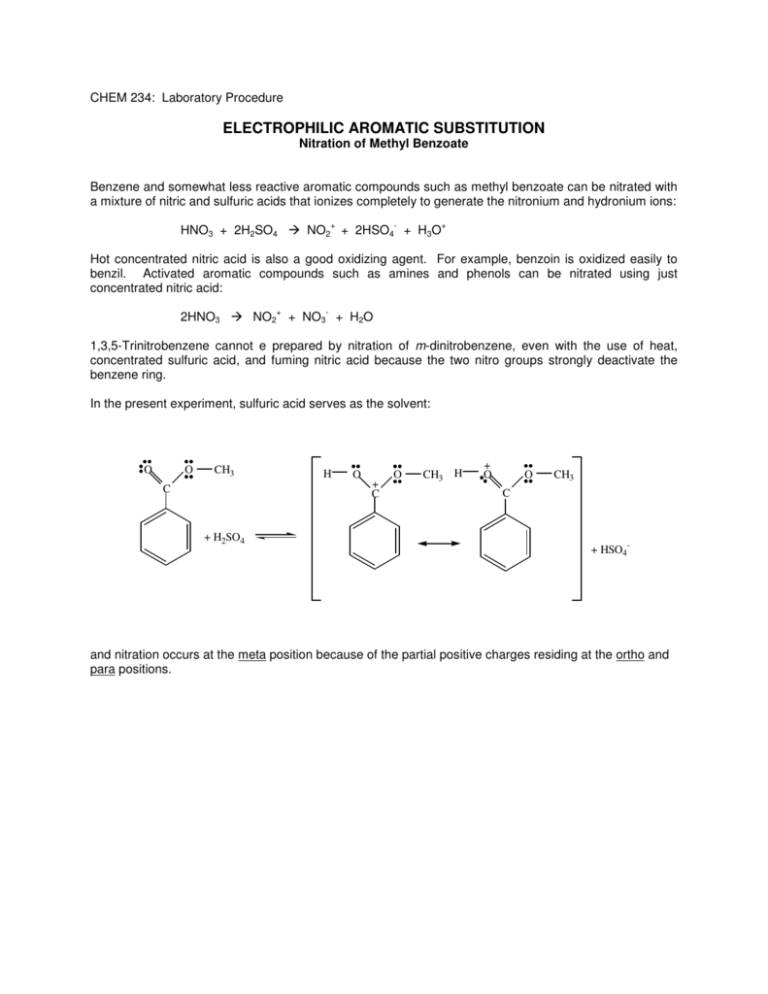
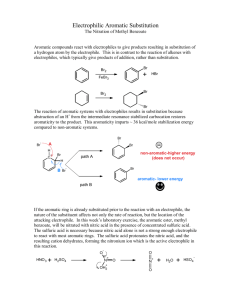
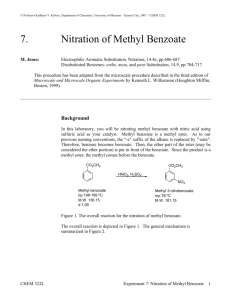
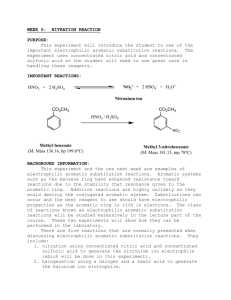
CHEM 234: Laboratory Procedure ELECTROPHILIC AROMATIC SUBSTITUTION Nitration of Methyl Benzoate Benzene and somewhat less reactive aromatic compounds such as methyl benzoate can be nitrated with a mixture of nitric and sulfuric acids that ionizes completely to generate the nitronium and hydronium ions: + - HNO3 + 2H2SO4 NO2 + 2HSO4 + H3O + Hot concentrated nitric acid is also a good oxidizing agent. For example, benzoin is oxidized easily to benzil. Activated aromatic compounds such as amines and phenols can be nitrated using just concentrated nitric acid: + - 2HNO3 NO2 + NO3 + H2O 1,3,5-Trinitrobenzene cannot e prepared by nitration of m-dinitrobenzene, even with the use of heat, concentrated sulfuric acid, and fuming nitric acid because the two nitro groups strongly deactivate the benzene ring. In the present experiment, sulfuric acid serves as the solvent: O O CH3 C + H2SO4 H O + C O CH3 H + O O CH3 C + HSO4- and nitration occurs at the meta position because of the partial positive charges residing at the ortho and para positions. Nitration of Methyl Benzoate NO2+ + 2HSO4- + H3O+ HNO3 + 2H2SO4 Nitronium ion COOCH3 Hydronium ion COOCH3 HNO3 H2SO4 NO2 Methyl benzoate MW 136.16 o Methyl 3-nitrobenzoate bp 199.6 C MW 181.15 den 1.09 mp 78oC In a 125-mL Erlenmeyer flask cool 12 mL of concentrated sulfuric acid to 0°C and then add 6.1 g of methyl benzoate. Again cool the mixture to 0-10°C. Now add dropwise, using a Pasteur pipette, a cooled mixture of 4 mL of concentrated sulfuric acid and 4 mL of concentrated nitric acid. During the addition of the acids, swirl the mixture frequently and maintain the temperature of the reaction mixture in the range of 5-15°C. When all the nitric acid has been added, warm the mixture to room temperature and after 15 min pour it on 50 g of cracked ice in a 250 mL beaker. Isolate the solid product by suction filtration using a small Buchner funnel and wash well with water, then with two 10-mL portions of ice-cold methanol. A small sample is saved for a melting point determination. The remainder is weighed and crystallized from an equal weight of methanol. Cleaning Up Dilute the filtrate from the reaction with water, neutralize with sodium carbonate, and flush down the drain. The methanol from the crystallization should be placed in the organic solvents container.