Aromatic Compound Naming & Structure: Organic Chemistry
advertisement

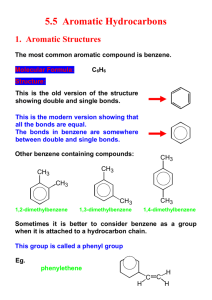
Naming Aromatic Compounds (Benzene as the Parent) • • If the the alkyl chain is smaller than the ring use the ring as the parent Monosubstituted benzenes named the same way as other hydrocarbons using –benzene as the parent name Aromatic Compounds CH3 CH3 CH3 CH3 H3C CH3 CH3 CH3 Common Name toluene benzene ortho-xylene methylbenzene IUPAC CH3 meta-xylene 1,2-dimethylbenzene cumene para-xylene 1,3-dimethylbenzene 1,4-dimethylbenzene isopropylbenzene Other aromatic compounds: OH Cl phenol chlorobenzene CH2OH O NH2 OMe aniline anisole H O OH SO3H NO2 benzene sulfonic acid O nitrobenzene CH3 biphenyl benzylalcohol benzaldehyde or phenylmethanol benzoic acid acetophenone HC CH2 styrene More Aromatic Compounds Polycyclic Aromatic Compounds 6 5 8 1 7 6 5 1 2 2 3 3 4 4 naphthalene 9 10 7 4 8 7 3 6 2 8 9 5 anthracene 1 10 phenanthrene 2,4,5,6,10-pentamethylphenanthrene Heterocyclic Aromatic Compounds O H N N furan pyrrole N N pyridine N pyrimidine N H indole Naming Aromatic Compounds (Disubstituted Benzenes) • Relative positions on a benzene ring • ipso- carbon 1 • ortho- (o) on adjacent carbons (1,2) • meta- (m) separated by one carbon (1,3) • para- (p) separated by two carbons (1,4) N H N N purine Naming Aromatic Compounds (Benzenes with More Substituents) • • • Choose numbers for the lowest possible values List groups alphabetically (hyphenated between) numbers Common names, such as toluene can serve as root name (as in TNT) Naming Aromatic Compounds (Benzene as a Substituent) • • If the the alkyl chain is larger than the ring use it as the parent When a benzene ring is a substituent, the term phenyl is used (for C6H5–) • • You may also see “Ph” or “φ” or “C6H5” Remember that benzyl refers to “C6H5CH2–” Structure of Benzene H H H H H H • All its C-C bonds are the same length (1.39 Å) • • • between length of normal single and double bonds Electron density in all six C-C bonds is identical Structure is planar Stability of Benzene (Heats of Hydrogenation) H2, Pd H2, Pd H2, Ni 175 °C 180 atm H2, Pd ΔH°exp = – 28.6 kcal/mol ΔH°est = – 57.2 kcal/mol ΔH°exp = – 55.5 kcal/mol = 1.7 kcal resonance energy ΔH°est = – 85.8 kcal/mol ΔH°exp = – 49.8 kcal/mol = 36 kcal resonance energy ΔH°est = – 85.8 kcal/mol ΔH°exp = – 80.6 kcal/mol = 5.2 kcal resonance energy Aromaticity and the 4n + 2 Rule 1 monocyclic cmp (i.e. don't look at anthracene, etc) 2 All atoms in the ring must have a p-orbital (sp2 atoms) 3 The p-orbitals must overlap...the cmp must be flat and the overlap unbroken 4 π-cloud must contain 4n+2 electrons (Hückel's Rule) n = 0,1,2,3,4,5.... Therefore, compounds with 2,6,10,14,18 electrons will be aromatic ALL the rules must be followed to be aromatic! Huge energy advantage to being aromatic! H H H H H sp3 H Antiaromatic Compounds 1 monocyclic cmp (i.e. don't look at anthracene, etc) 2 All atoms in the ring must have a p-orbital (sp2 atoms) 3 The p-orbitals must overlap...the cmp must be flat and the overlap unbroken 4 π-cloud must contain 4n electrons n = 0,1,2,3,4,5.... Therefore, compounds with 4,8,12,16,20 electrons will be antiaromatic ALL the rules must be followed to be antiaromatic! Basically, nature avoids...use to explain why stuff doesn't happen H H X H H H H Aromatic Ions Aromatic Heterocycles • A Heterocycle is a cyclic compound that contains an atom or atoms other than carbon More Aromatic Heterocycles O Furan Thiophene Special Cases Pay Attention! O O N CH3 X N H3C H N N N N H H Special Compounds With 4n π Electrons 4π electrons antiaromatic Exists @ 4 K What is Aromaticity? MO Description of Benzene Frost Diagrams Inscribe the ring in a concentric circle with one of the points down Draw energy levels at each of the intersections between the circle and the ring Cut circle in half Fill the levels with π electrons starting at the bottom and working your way up 1. 2. 3. 4. 6πe non-bond MO's bonding MO's Why 4n+2? • Aromatic NB • NB Antiaromatic or Nonaromatic NB NB