The basic trigonal bipyramidal molecular
advertisement
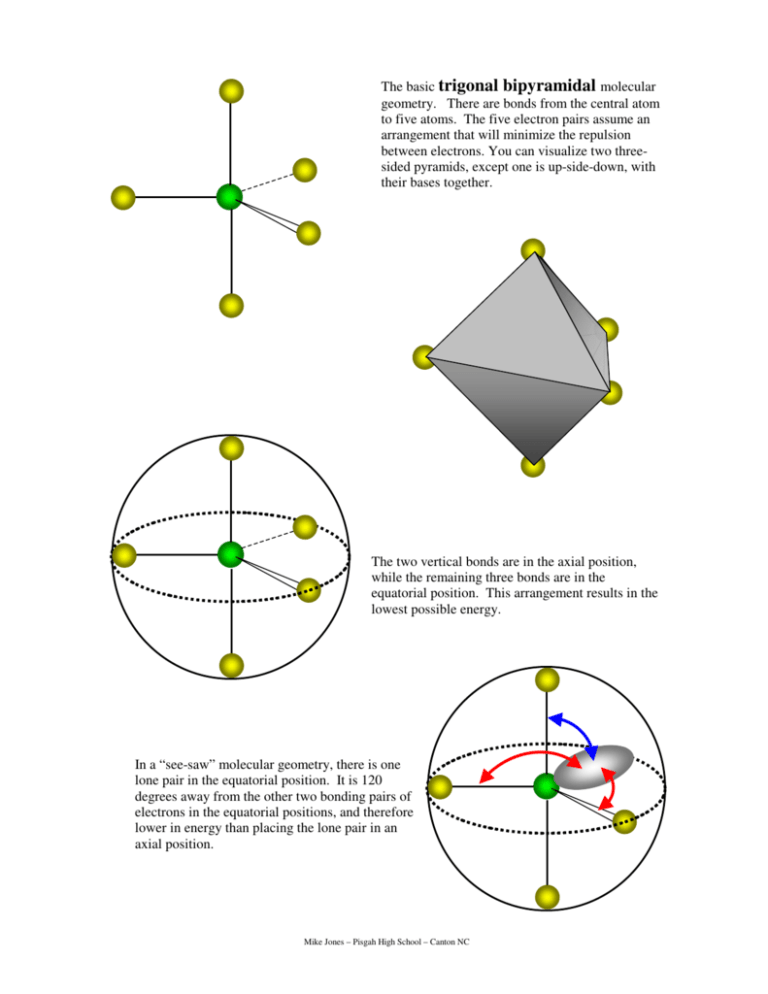
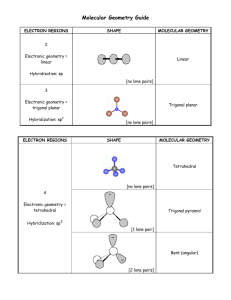
The basic trigonal bipyramidal molecular geometry. There are bonds from the central atom to five atoms. The five electron pairs assume an arrangement that will minimize the repulsion between electrons. You can visualize two threesided pyramids, except one is up-side-down, with their bases together. The two vertical bonds are in the axial position, while the remaining three bonds are in the equatorial position. This arrangement results in the lowest possible energy. In a “see-saw” molecular geometry, there is one lone pair in the equatorial position. It is 120 degrees away from the other two bonding pairs of electrons in the equatorial positions, and therefore lower in energy than placing the lone pair in an axial position. Mike Jones – Pisgah High School – Canton NC In a T-shaped molecule, the lone pairs are directed towards the “equator”. This arrangement places each lone pair at right angles to the axial bonding pairs, and 120 degrees away from the other lone pair in the equatorial position, and 120 degrees away from the bonding pair in the equatorial position. This results in lower overall energy. If the lone pairs were in the axial positions, the molecule would be trigonal planar. But the lone pairs would be 90 degrees from all three of the bonding pairs. This would result in greater repulsion and a system at a higher energy than a T-shaped molecule. Therefore, the T-shaped molecule is preferable. In a linear molecule, all three lone pairs are directed towards the “equator”. This arrangement places each lone pair at right angles to the axial bonding pairs, and 120 degrees away from the other lone pairs in the equatorial position. This results in lower overall energy. Mike Jones – Pisgah High School – Canton NC Review exercises. Answer on notebook paper. Make your answers complete and to the point. 1. Explain why the bond angles in the trigonal bipyramidal electron pair geometry are 90 degrees and 120 degrees. 2. Explain the difference between the terms “axial” and “equatorial” as it applies to molecular geometry. 3. A student suggested that PCl5 could have bond angles of 72 degrees. Describe the arrangement of the electron pairs in the molecule that the student was imagining? How would you explain to that student why the bond angles are not 72 degrees? 4. What allows phosphorous to have five bonds, anyway? After all, there are only three unpaired electrons in the 3p-sublevel. (Hint: Draw the electron energy diagram of phosphorous.) 5. Assuming that phosphorous can have five bonds, explain why nitrogen, which is in the same family on the periodic table, cannot have five bonds. 6. Consider BeCl2, SCl2 and XeCl2. Determine which one(s) are linear and explain why. 7. Explain why ICl3 is T-shaped and not trigonal planar. 8. Explain why in a T-shaped molecule the two electrons pairs in the axial positions are not exactly 180 degrees apart, while both are slightly less than 90 degrees from the one equatorial bonding pair. 9. Draw the Lewis diagrams for CCl4, SCl4 and XeCl4 and give the molecular geometry of each. Explain what is different about each of the molecules that determines the molecular geometry. 10. Consider the behavior of lone pairs of electrons as you explain why the ICl4- ion has a molecular geometry of square planar and not see-saw.