Quiz 4 KEY
advertisement

1 (10 POIns
. t ) Compee
I t th e f 0 IIowinq t a bl e:
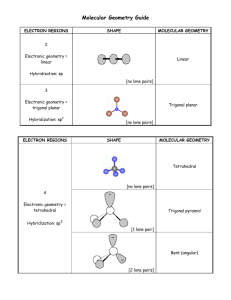
Electron Geometry
# Electron
Groups
to
o ctC\.Y\ea.~\
4
# lone
pairs
Molecular Geometry
Hybridization
d-
Square Planar
S?3d'L-
'\ '-W C\'xe:c...'<0..\
2
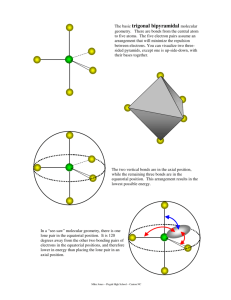
Trigonal Bipyramidal
2
0?~
be'CYt
T-s\\q?ec\
V)
S?~C\
2.(10 points) Answer the following questions about
3,4-methylenedioxy-N-methylamphetamine),
a psychoactive drug most commonly known
today by the street name "Ecstasy".
H
H
/~--c~
~c
/
H
I
H
I
C
H5
N
~c~
II
2
\
H
\/
c
1
,0
H
/
"c~3~C--H
I
/ -,
H
O°"--C"C~C"o.
4\
C__
H
/ \
H H
H
H
I
H
(a) The lone pairs have not been shown in the molecule. How many lone pair electrons
(5
t (j e-
are in Ecstacy?
\ C'0e
(b) What is the hybridization at Carbon #1?
S? 2-
(c) What is the hybridization at Nitrogen #3?
S?~
yG.\ \'"5 )
(d) What is the hybridization at Carbon #4?
S £ ':>
(e) What is the hybridization at Oxygen #2?
S? ~
(f) What is the electron geometry at Carbon #1 ?
\3 G00\
j '(
"? \0.. Y1o..'('
(g) What orbitals are used to make the sigma bond between Carbon#4 and
Hydrogen#5?
C
(h) The H5- C4
-
(S
'6
-
s)
\
H bond angle is circle one
(>0= )
(i) What is the molecular geometry at Nitrogen #3?
to
6 q .S ~
'.
.1'<\86D(1\ ~j'(o.\'Y\ \QC\.\
(k) How many pi bonds are present in Ecstacy?_'-.",,"-3~
2
\
_
3.
(4 points) You have used oxalic acid, C2H204, several times in lab. The skeletal
(incomplete) structure is shown below.
(a) Fill in all of the missing lone pairs (if applicable) and double bonds (if applicable).
:0:
:0··
H-o-~-l\-·o·-H
.
,
(b) Recall that oxalic acid is a diprotic acid. The oxalate ion, (the conjugate base of
oxalic acid) is stabilized by resonance structures. Write out the lewis structure of the
oxalate ion, including all of its resonance structures.
C'2..
L--
0 L1
4. (6 points) Starting with the best Lewis structure, write out all of the equivalent
resonance structures for the sulfate anion. Label the formal charge on each atom. Hint:
in the best lewis structure, sulfur will expand its octet in order to have a formal charge of
zero.
5. (4 points) Consider the following molecular geometry. What is the electron geometry
of this molecule?
\
"->
3
see so,uJ
6. (4 points) Draw the Lewis dot structure for the compound formed between strontium
and nitrogen.
7. (8 points) Answer the following questions about the molecule, SCI4•
(a) Draw the VSEPR structure of this compound, indicating any 3D shape using dashes
and wedges. Identify any polar bonds by drawing this arrow beside them: +--+
(0
-t -::r (t\) -=3L\ e-
t
Q
(b) This molecule is (Circle one)
moment (show this by drawing th~ole
nonpolar. What direction is the net dipole
arrow above.
(c) What is the molecular geometry of this molecule?
Se.e5P.. W
(d) What is the electron geometry of this molecule?
W\f3<J'('O \
'9\ ~~'(o..'(Y\\ ClC\.\
8. (4 points) What is the bond order of Hel+? Show your work below.
4