Molecular shapes_VSEPR(download)
advertisement

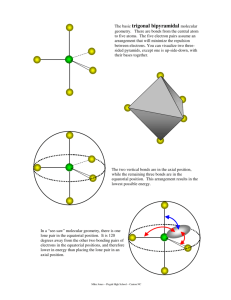
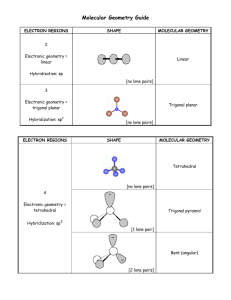
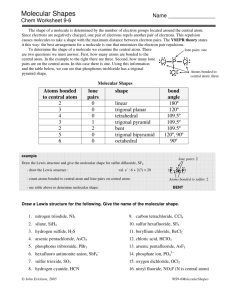
Molecular shapes A simple matter of balls and sticks Learning objectives Describe underlying principles that govern theories of molecular shapes Use Lewis dot diagrams to predict shapes of molecules using VSEPR Valence shell electron pair repulsion In order to understand properties like polarity, we need to predict molecular shapes Lewis dot structure provides 2D sketch of the distribution of the valence electrons among bonds between atoms and lone pairs; it provides no information about the shape of the molecule A hierarchy of models VSEPR Consider the problem in terms of electrostatic repulsion between groups of electrons (charge clouds, domains) Valence bond theory Acknowledges the role of orbitals in covalent bonding Molecular orbital (MO) theory (the “real” thing) Accommodates delocalization of electrons - explains optical and magnetic properties Electron groups (clouds) minimize potential energy Valence shell electron pair repulsion (VSEPR) Identify all of the groups of charge: non-bonding pairs and bonds (multiples count as one) Distribute them about the central atom to minimize potential energy (maximum separation of the groups) This specifies the electronic geometry (also known as electron domain geometry or sometimes confusingly as molecular geometry) Choices are limited Groups (domains) of charge range from 2 – 6 Only one electronic geometry in each case However, more than one molecular shape follows from electronic geometry depending on number of lone pairs One surprise: the lone pairs occupy more space than the bonded atoms (with very few exceptions) Manifested in bond angles (examples follow) Molecular shape selection (particularly in trigonal bipyramid) Two groups: linear Except for BeH2 (Be violates octet rule), all cases with two groups involve multiple bonds Three groups: trigonal planar Two possibilities for central atoms with complete octets: Trigonal planar (H2CO) Bent (SO2) BCl3 provides example of trigonal planar with three single bonds B is satisfied with 6 electrons – violates octet rule Four groups: tetrahedral Three possibilities: No lone pairs (CH4) tetrahedral One lone pair (NH3) – trigonal pyramid Two lone pairs (H2O) – bent Lone pairs need space: • H-N-H angle 107° • H-O-H angle 104.5° • Tetrahedral angle 109.5° Representations of the tetrahedron Five groups of charge: trigonal bipyramid – most variations Two different positions: Three equatorial Two axial Equatorial positions are lower energy: Lone pairs require occupy these locations preferentially Five bonds, no lone pairs Four bonds, one lone pair Lone pair dictates geometry: equatorial position has lower energy than axial Three bonds, two lone pairs Both lone pairs occupy equatorial positions – lower energy than in axial Two bonds, three lone pairs The trend continues: all equatorial positions filled – lowest energy Octahedron has six identical positions and high symmetry No lone pairs High symmetry One lone pair All positions are equally probable Symmetry reduced Two lone pairs Minimum energy has axial symmetry, lone pairs lie along straight line Molecules with multiple centers A central atom is any atom with more than one atom bonded to it Perform exercise individually for each atom Electronic geometry and molecular shape will refer only to the atoms/lone pairs immediately attached to that atom Taking it to the next level: acknowledging orbitals VSEPR is quite successful in predicting molecular shapes based on the simplistic Lewis dot approach But our understanding of the atom has the electrons occupying atomic orbitals How do we reconcile the observed shapes of molecules with the atomic orbital picture of atoms