Hansen 1 Zac Hansen Chem 312L R8am 5/9/2013 Benzocaine
advertisement

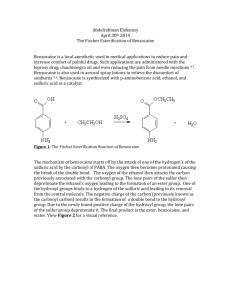
Hansen 1 Zac Hansen Chem 312L R8am 5/9/2013 Benzocaine Introduction The purpose of this experiment is to perform a fischer esterification. The reaction makes benzocaine, a topical anesthetic, from EtOH, p-aminobenzoic acid, and sulfuric acid as a source of H+. The reaction taking place lies in equilibrium so the source of H+, sulfuric acid, needs to be present to catalyze the reaction. To push the equilibrium to the right, an excess of EtOH is used so more product is formed. Since the amino group present on Benzocaine is basic and will therefore use up some of the protons, sodium carbonate is introduced after reflux to deprotonate the final product. Benzocaine is similar to the majority of anesthetics in that it contains an aromatic ring, short chain of carbons, and an amino group. The presence of amino groups is thought to aid binding and solubility. Most anesthetics contain tertiary or secondary amino groups and since Benzocaine possesses only a primary amino group it is used only for topical treatments. Benzocaine is most commonly used in sunburn relief. Reaction Summary Procedure Reaction 0.616g of p-aminobenzoic acid was combined with 6 mL of dry EtOH into a 25 mL round bottom flask. Boiling stones were added to the flask as well. The solid was stirred until Hansen 2 completely dissolved. 0.5 mL of concentrated sulfuric acid was added drop wise to the flask and stirred. The flask was set up for reflux with a water cooled condenser and refluxed for 75 minutes. Isolation After the reflux period was complete, the reaction mixture was allowed to cool. The mixture was transferred to a flask containing 15 mL of water. When the reaction mixture had reached room temperature, 5+ mL of 10% Na2CO3 was added drop wise to the mixture until it was sufficiently basic, pH ≥ 8. The flask was then placed into an ice bath and then the crystals were isolated through vacuum filtration. The product was allowed to dry and then a weight, melting point, and 1H NMR spectrum was obtained. Results Table 1. Experimental yield and melting point data for benzocaine Compound Amount used Exp yield Theo yield % yield Exp MP Lit MP p-aminobenzoic acid 0.616 g Sulfuric Acid 0.5 mL EtOH 6 mL Benzocaine 0.023 g 0.742g 3.10% 75-78 °C 88-90°C* *Sigma-Alderich. Material Safety Data Sheet. Page 3 of 7. Last revision date: 11/13/2012. Web, 05/07/2013. Table 2. 1H NMR data of benzocaine H’s of that type Splitting 2 Doublet 2 Doublet 4* Quartet, Broad Singlet 3 Triplet *Two types together Chemical shift 7.8ppm 6.6ppm 4.6ppm 0.4ppm Indication Aryl Aryl Near C-O, NH2 Alkyl Hansen 3 Figure 1. 1H NMR spectrum of benzocaine Discussion The experiment overall was poor. The percent yield was extremely low, with only 3.10% product yield. The low yield can be attributed to an inadequate amount of H+ in the reaction mixture. Along with a very low yield, the melting point of the compound was also very low, 7578 °C compared to its literature value of 88-90 °C. The 1H NMR spectrum of benzocaine was not obtained experimentally, so spectrum is a representation of a more pure compound. The 1H NMR spectrum shows four peaks. The first peak (from left to right) represents 2 hydrogens and has a chemical shift of about 7.8 ppm with 1 neighboring hydrogen. The second peak represents 2 hydrogens that have a chemical shift of 6.6 ppm and one neighboring hydrogen. This evidence is consistent with a p-substituted compound. The third peak is a bit tricky because it actually contains two types of hydrogens. The peak has a chemical shift of about 4.6 ppm, so one type of hydrogen involved is near a C-O bond. There are 2 H’s near the C-O bond. These 2 hydrogens Hansen 4 have 3 neighboring hydrogens which are the alkyl hydrogens. The remaining portion of the third peak is a broad singlet representing the hydrogens bonded to the nitrogen. The fourth peak, farthest to the right, has a chemical shift of about 0.4 ppm with 2 neighboring hydrogens. This peak represents the 3 alkyl hydrogens neighboring the 2 hydrogens near the C-O bond. Conclusion The simplicity of this experiment did not leave much room for error; however there was one major error that occurred within the experiment. The inadequate amount of H+ in the reaction mixture would result in very small amount of the electrophile needed for the reaction to occur. Since the lone pair present on the nitrogen is basic and more basic than the lone pairs on the oxygen, the nitrogen will be protonated first. If not enough protons are present in the reaction mixture, all of the amino groups will be protonated and not all the carbonyls would be. The yield would be low because majority of the carbonyls have not been protonated. EtOH, a weak nucleophile, needs assistance to attack a weak electrophile, the carbonyl, and still get a decent yield. This experiment was useful in providing a first-hand experience of performing a Fischer esterification and its mechanism. The experiment was also useful in demonstrating the importance of using a catalyst when a reaction with a weak nucleophile and a weak electrophile takes place.