The Fischer Esterification of Benzocaine
advertisement

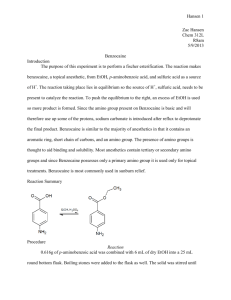
Abdelrahman Elshamsy April 30th 2014 The Fischer Esterification of Benzocaine Benzocaine is a local anesthetic used in medical applications to reduce pain and increase comfort of painful drugs. Such applications are administered with the leprosy drug, chaulmoogra oil and even reducing the pain from needle injections 4 7. Benzocaine is also used in aerosol spray lotions to relieve the discomfort of sunburns 3 6. Benzocaine is synthesized with p-aminobenzoic acid, ethanol, and sulfuric acid as a catalyst. Figure 1: The Fischer Esterification Reaction of Benzocaine The mechanism of benzocaine starts off by the attack of one of the hydrogen’s of the sulfuric acid by the carbonyl of PABA. The oxygen then becomes protonated causing the break of the double bond. The oxygen of the ethanol then attacks the carbon previously associated with the carbonyl group. The lone pairs of the sulfur then deprotonate the ethanol’s oxygen leading to the formation of an ester group. One of the hydroxyl groups binds to a hydrogen of the sulfuric acid leading to its removal from the central molecule. The negative charge of the carbon (previously known as the carbonyl carbon) results in the formation of a double bond to the hydroxyl group. Due to the newly found positive charge of the hydroxyl group, the lone pairs of the sulfur group deprotonate it. The final product is the ester, benzocaine, and water. View Figure 2 for a visual reference. Figure 2: The mechanism walkthrough of the esterification of benzocaine. Experimental: Benzocaine from PABA, ethanol, and sulfuric acid. Benzocaine. 4-aminobenzoic acid (250mg) and ethanol (3mL, 100%) were added to a round bottom flask (25mL) and stirred under a heated sand bath until dissolved. Mixture is cooled in ice and sulfuric acid (0.40 mL) is added dropwise. Mixture is then refluxed for 70 minutes. After cooling to room temperature, distilled water is added to the reaction mixture in a beaker (50mL). Sodium bicarbonate (8mL) is then added dropwise to the reaction mixture until the pH of the solution is more than 8. Crude white crystals of benzocaine are collected by vacuum filtration and recrystallized (100% ethanol, hot water) after drying the next lab period. Final purified product was vacuum filtered and dried as a white crystalline solid (192mg; 76.8%); pH (8.0-9.0); MP (86-88°C); IR (cm-1) 1115.72, 1679.56, 2983.68, 3221.11, 3340.71, 3419.59; GC (benzocaine, 40°C to 250°C at 10°C per min) RT 14.20; GC-MS (phenyl methylpolysiloxane, 40°C to 250°C at 10°C per min) RT 18.95, 165m/z. 1HNMR (60 MHz, DMSO) (ppm), 1.268 (t, 3H), 4.140 (q, 2H), 5.935 (s, 2H), 6.605 (d, 2H), 7.573 (d, 2H). Discussion and Conclusion: Benzocaine, an ester, was synthesized from p-aminobenzoic acid, absolute ethanol, and sulfuric acid. The synthesis of benzocaine is an important procedure because it is a common anesthetic with many uses in the medical and is also a learning experience of Fischer esterification processes. In this reaction, sulfuric acid plays a huge role as a catalyst and is necessary for this reaction to undergo. The nucleophilic substitution of the reaction results in the change between the –OH group of the carboxylic acid to an –OCH2CH3 group. The yielded amount was 192 mg or 76.8% yield that fell short of the European Polymer Journal article which was at 90% yield2. An explanation to the slightly lower yield is from lost product through transfers (from beaker to watch glass, etc) as well as product adhered to the filter papers from the vacuum filtration processes done twice. Purification by recrystallization was a successful process as there were no starting materials or intermediates in the mixture shown by 1HNMR, IR, GC, MP and GC-MS data. However, there was evidence of an impurity along with the product. The melting point data had a slightly lower range than the observed range on sigmaaldrich.com, but however, the acquired range matched the minimum temp from the observed range on sigmaaldrich.com (88-90°C)8. The impurity is believed to be water and is seen on the 1HNMR data. Its presence may have possibly occurred from a product that was not fully dried. 1HNMR data showed the presence of benzocaine without starting materials. There are peaks in the data that are solely present in benzocaine and is as follows. At the triplet peak 1.268 with integral value of approximately 3 is the peak for the –CH3 group (Ha) in its proper position. Quartet peak at 4.140 with the integral value of 2 is for the –CH2 group that is attached to the oxygen and methyl group. In the 1HNMR results, the hydroxyl hydrogen for the carboxylic acid is not present. This peak would have a singlet splitting patter and would be found at the 11.0-12.0 range. The hydroxyl hydrogen of the ethanol also does not show up on the 1HNMR data, which would be a doublet peak at 0.5-5.0 and an integral value of 1. 1HNMR spectra of benzocaine on sigmaaldrich.com confirm the correctness of the 1HNMR spectra acquired8. The IR data is not very much different of that of the starting materials besides for one peak. The IR data is a good reference however for the presence of the product, benzocaine. All of the significant peaks are present at 3419.59 for the –NH stretch, 3340.71 and 3221.11 for the benzyllic –CH stretch, 2983.68 for the –CH stretch, 1679 for the C=O stretch, and 1115.72 for the C-O stretch. The only difference in this IR data from the IR data of the starting materials is the presence of the ester at 1115.72, which is clearly not present in starting materials as it was the highlight of this reaction. IR spectra of Fluorescein on sigmaaldrich.com confirm the correctness of the IR spectra acquired8. GC data showed one significant peak at 14.20 (retention time) with area percentage at 100%. GC-MS data also showed one peak at 18.95 (retention time) with area percentage of 100% and 165 m/z. The GC and GC-MS data both showed that the single peak at 14.20 & 18.95 is in fact for benzocaine from the library database. Overall, the product was successfully acquired. The drying time could’ve been increased to remove the water and acetone as seen in the 1HNMR and the depressed melting point but due to time constraints this wasn’t an option during this attempt. The IR, melting point, GC, GC-MS and 1HNMR all correlated to one another and showed that the product was successfully present with little impurities. References: 1. Demare, P. & Regla, I. Synthesis of Two Local Anesthetics from Toluene: An Organic Multistep Synthesis in a Project-Oriented Laboratory Course. J. Chem. Educ. 2012, 89, 147–149. 2. European Polymer Journal 2013, 49 , 2247 3. Gilpin, R. K., Pachla, L.A., & Ranweller, J.S.. Pharmaceuticals and Related Drugs. Anal. Chem., 2002, 55 (5), pp 70R–87R 4. Nusstein, J. M. & Beck, M. Effectiveness of 20% benzocaine as a topical anesthetic for intraoral injections. Anesth Prog. 2003; 50(4): 159–163. 5. Rummel, A. S., Ed. Lab Guide for Chemistry 213; Hayden Mcneil; University Park, PA, 2013. 6. Rummel, A. S. Experiment 16: The Fischer Esterification of Benzocaine 2013. The Pennsylvania State University ANGEL Course Spring 2014 Chem 213 Web site. 7.Science Service. Leprosy Drug Now Administered Painlessly. J. Chem. Educ., 1928, 5 (4), p 444 8. Sigma Aldrich. Chemical database. www.sigmaaldrich.com. Mar 23rd 2014.