Experiment K - Georgia Gwinnett College
advertisement

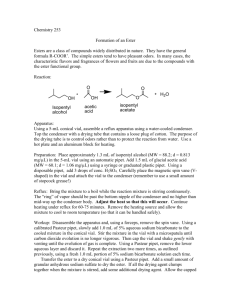
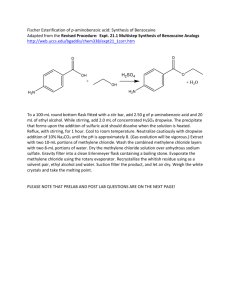
Organic Chemistry II Lab CHEM 2212L (Fall 2008) School of Science and Technology Georgia Gwinnett College Experiment K. Fischer Esterification Reaction of a Carboxylic Acid Derivative: Preparation of Benzocaine 1. EXPERIMENTAL OBJECTIVES: a. Conduct an acid-catalyzed esterification of a carboxylic acid with an alcohol. b. Isolate the product. c. Characterize and analyze. d. Present, analyze, and discuss results in a written report. 2. SPECIAL INSTRUCTIONS: a. Chemicals and solvents: (1) p-aminobenzoic acid, [150-13-0] (2) absolute ethanol, [64-17-5] (3) concentrated sulfuric acid, [7664-93-9] (4) 10% aqueous sodium carbonate, [497-19-8] (5) methanol, [67-56-1] (6) benzocaine (ethyl p-aminobenzoate), [94-09-7] b. Your goal in the first lab session is to completely finish the experimental procedures. You must be organized and ready to work in order to do so. The second period is for analysis. 3. PROCEDURES: Apparatus 1. You do not need the drying tube shown in the sketch in G&M on p. 657. Use the smallest bar magnet. Setting Up 2. Weigh the p-aminobenzoic acid and use the ADP to dispense the 2.0 mL of absolute ethanol. Connect your vial to the condenser, but do not heat yet, and stir the mixture until everything goes into solution. Add the 0.2 mL (approximately 10-12 drops) of concentrated sulfuric acid with a glass pipet through the top of the condenser. Reaction 3. After the acid is added, begin heating to a gentle reflux (about a heat setting of 4). Get the best stirring action you can. After refluxing for 20 minutes, raise the apparatus out of the sand bath and with the condenser still connected and the water running, hold a beaker of water under the conical vial to aid cooling to room temperature. Solid will most likely Page 1 of 2 2/16/16 Organic Chemistry II Lab CHEM 2212L (Fall 2008) School of Science and Technology Georgia Gwinnett College remain, so after the vial is cool, uncap it and add 0.500 mL absolute ethanol with the ADP. Recap the vial to the condenser and add another 5-6 drops of concentrated sulfuric acid. Lower the apparatus back into the sand bath and continue refluxing for another 30 minutes. Ensure you are refluxing gently and stirring during this 30 minutes. 4. While the 30 minute reflux is ongoing, set up equipment for the remainder of the experiment, cool about 20 mL (use two centrifuge tubes) of distilled water in an ice bath for use in both the work-up and purification steps, and do a quasi rehearsal of the remaining procedures of the lab. If time permits in the 30 minutes, do an IR of p-aminobenzoic acid and absolute ethanol, print them in a stacked view spectrum. If time does not permit the IRs, you will do them in the second lab period. Work-Up and Isolation 5. It will take about 4-7 mL of 10% sodium carbonate to bring about the pH change. Measure the pH before you begin the addition of sodium carbonate and then after about each mL you add. Swirl the mixture in the beaker to ensure mixing, but be careful not to slosh. 6. No need to air dry the product at the end of the Work-Up and Isolation section. Immediately place the crude, wet product into a 25 mL Erlenmeyer flask and begin the purification. Purification 7. You do not need to weigh your crude product. Proceed directly to the recrystallization. 8. Use a thermometer to get the 60 oC temperature. You don’t need to heat very much. Do not go above this temperature. You will then add 1-2 mL of methanol. 9. Place your recrystallized product into an uncapped, labeled sample vial until the second lab period. Analysis 9. Determine percent yield of recrystallized product; IR of p-aminobenzoic acid, ethanol, and the purified benzocaine product; and melting point of the purified product. Page 2 of 2 2/16/16