Benzocaine Synthesis: Fischer Esterification Lab Experiment
advertisement

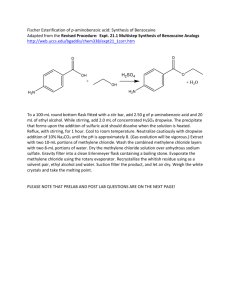
CH421 Experiment 4 Synthesis of Benzocaine via Fischer Esterification of para-Aminobenzoic Acid Reading: Organic Chemistry by John McMurry, 8e, Chapter Sections 21.3 and 21.6 Techniques: IR, NMR Introduction Carboxylic acid esters (or just esters) have the general formula RCO2R', where R and R' may be an alkyl or an aryl group. Some esters are flavoring or odor concentrates, while others have medicinal uses. Esters may be formed by the direct reaction of a carboxylic acid with an alcohol. This reaction is called Fischer esterification, which is both acid-catalyzed and reversible. The acid catalyst, generally a mineral acid, protonates the carbonyl group of the carboxylic acid making it more readily attacked by the nucleophilic oxygen of the alcohol. After proton transfer, a molecule of water is then eliminated to form the ester. At reflux temperatures and in the presence of an acid catalyst, both the forward and reverse esterification reactions are rapid, and the system reaches equilibrium rather quickly. In order to obtain a high yield of ester, however, the equilibrium must be shifted toward the products (Le Chatelier’s Principle). One technique for accomplishing this is to use a large excess of one of the reactants (usually the alcohol). Another method is to remove one (or more) of the products as they are formed. This can be accomplished by physical (e.g., azeotropic distillation of water) or chemical means. The position of equilibrium is determined by the equilibrium constant (K), as defined by the following mass-action expression: K = The equilibrium constants for the formation of a couple different esters of acetic acid from the corresponding alcohols in the presence of an acid catalyst are given in the table below. The K values were determined experimentally from systems at equilibrium and may be taken as a measure of the efficiencies of the esterification processes. For example, the values indicate that ethyl acetate (K = 3.96) is prepared much more efficiently than is t-butyl acetate (K = 0.0049). In general, primary alcohols are comparatively easy to esterify, while secondary alcohols are esterified with some difficulty, and tertiary alcohols hardly react. K values for the formation of esters of acetic acid Alcohol ethanol t-butanol Ester Equilibrium Constant (K) ethyl acetate 3.96 t-butyl acetate 0.0049 Experiment 4: Synthesis of Benzocaine In this laboratory, the local anesthetic benzocaine is synthesized by Fischer esterification of para-aminobenzoic acid (PABA) (see scheme below with the reaction table). The Fischer esterification is normally catalytic in acid, since the acid is regenerated in the mechanism. However, if there is/are basic group(s) present in the substrate, stoichiometric acid is required since the basic group will consume the acid catalyst, as is the case in the esterification of PABA. In the workup of this reaction, aqueous Na2CO3 affords benzocaine in its free base form (the pKa of protonated benzocaine is 2.8). The water-insoluble product is thus readily isolated by filtration. Safety Precautions Ethanol is flammable. Sulfuric acid is extremely caustic. Safety glasses and gloves are mandatory. If any acid gets onto your skin, wash with excessive water. Pre-Lab Notebook (to be completed prior to the start of lab) • • Enter date, experiment number and title on notebook page and in TOC with corresponding page number. Copy the reaction and the reaction table below (in your own writing) into your notebook and complete all empty cells in the table. Make sure to check your calculations with your instructor before starting the experiment. d (g/mL) vol (mL) _____ ___ 46.07 0.789 12.0 ____ 98.08 1.84 1.0 ____ _____ ___ Lit. mp = Name MW (g/mol) mass (g) p-aminobenzoic acid 137.17 1.2 ethanol sulfuric acid ethyl-4-aminobenzoate (benzocaine) 165.19 Theor. mass = mmol Theor. mmol = equiv ____ mp (°C) bp (°C) ____ ___ Experiment 4: Synthesis of Benzocaine Procedure Fischer Esterification To a 100 mL round-bottom flask equipped with a magnetic stir bar, add 1.2 g of p-aminobenzoic acid and 12.0 mL absolute ethanol. Stir the mixture until the solid dissolves. Slowly add 1.0 mL of concentrated H2SO4 using a glass pipette to transfer the acid. A precipitate is expected to form. Attach a reflux condenser to the flask, secure the apparatus with clamps, and heat the mixture at a gentle reflux for 60-75 minutes using a heating mantle and Variac power controller. The solid should dissolve as it undergoes reaction. Reaction Workup After completion of the reflux, allow the reaction mixture to cool to room temperature. Pour the reaction mixture (along with the stir bar) into a 200 mL beaker containing 30 mL of ice water. While stirring this mixture, slowly add approximately 10 mL of a 10% Na2CO3 solution. Gas evolution will be observed as the acid is neutralized; the final pH should be approximately 8. Vacuum filter the resulting precipitate, and wash the product with three 10 mL portions of water. Empty the filtrate into the aqueous waste container #1, then replace the empty Erlenmeyer flask and continue pulling a vacuum on the product to allow it to dry for ca. 15 mins. Obtain the mass of product and calculate the percent yield. Record the IR spectrum, and obtain the 1H NMR spectrum of the product in CDCl3 (approximately 15 mg dissolved in ~ 0.75 mL CDCl3). Waste Aqueous Waste #1: the aqueous filtrate. Organic Waste #2: NMR solution and all acetone rinses (glassware, sidearm flask with crude product, NMR tube, etc.) Solid Chemical Waste #4: filter paper Data (collect and record in your notebook during the lab) • • • Describe product, give mass of product isolated, and calculate % yield (show calculation). Obtain IR (solid is best), staple in notebook, and assign major functional group peaks. Obtain 1H NMR spectrum (CDCl3), label as “Name and Name Expt 4 prod in CDCl3,” and staple into notebook. Draw the structure of the product on the NMR spectrum, and label all peaks. Conclusion (record in your notebook) • State outcome, yield, proof of success and purity, possible sources of error, and any other information deemed necessary and relevant. Experiment 4: Synthesis of Benzocaine The IR and 1H NMR spectra for the PABA starting material are given below for your reference. IR of p-aminobenzoic acid (nujol mull) 1 H NMR of p-aminobenzoic acid (90 MHz, DMSO-d6) d, 2H d, 2H br s, 2H br s, 1H