File
advertisement

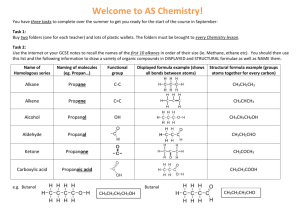
Organic Chemistry Organic chemistry concerns the chemical and physical properties of molecules in which the predominant element by percent composition is carbon. Most of organic chemistry is constructed from an investigation into the reactions of distinct clusters of elements arranged in specific ways called functional groups. Alkanes: molecules whose only constituent elements are C and H. Alkanes possess only single bonds between two carbon atoms, or between one carbon and one hydrogen atom. Students must become familiar with the names and structures of the ten simplest alkanes because the names of most other organic molecules depend upon them. General Formula: CnH2n+2 where n = 1, 2, 3 etc. Simplest Alkanes: methane CH4 ethane C2H6 or CH3CH3 propane C3H8 or CH3CH2CH3 CH3CH2CH2CH3 butane C4H10 or pentane C5H12 or CH3CH2CH2CH2CH3 hexane C6H14 or CH3CH2CH2CH2CH2CH3 heptane C7H16 or CH3CH2CH2CH2CH2CH2CH3 octane C8H18 or CH3CH2CH2CH2CH2CH2CH2CH3 nonane C9H20 or CH3CH2CH2CH2CH2CH2CH2CH2CH3 decane C10H22 or CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 Alkenes: molecules whose only constituent elements are C and H. Alkenes possess, in addition to single bonds between two carbon atoms, or between one carbon and one hydrogen atom, at least one double bond between two carbon atoms. General Formula: CnH2n where n = 2, 3, 4 etc. Simplest Alkenes: Alkenes are named after their parent alkanes with the same number of carbons, except that instead of the name ending with "-ane," it ends with "-ene." Note: It is impossible to have an alkene corresponding to methane. Therefore, have: ethene, propene, butene, pentene, hexene, heptene, octene, nonene, and decene. To indicate where the double bond(s) is/are placed, number the carbons from one end of the molecule and use the lowest numbered carbon having the double bond as part of the compound name. Indicate multiple double bonds with prefixes (di, tri, tetra, etc; the same prefixes used in inorganic compound names). examples: CH3CH=CHCH2CH2CH3 is 2-hexene, not 4-hexene CH2=CHCH2CH=CHCH2CH2CH2CH3 is 1,4-nonadiene Alkynes: molecules whose only constituent elements are C and H. Alkynes possess, in addition to single bonds between two carbon atoms, or between one carbon and one hydrogen atom, at least one triple bond between two carbon atoms. General Formula: CnH2n-2 where n = 2, 3, 4 etc. Simplest Alkynes: Alkynes are named after their parent alkanes with the same number of carbons, except that instead of the name ending with "-ane," it ends with "-yne." Note: It is impossible to have an alkyne corresponding to methane. Therefore, have: ethyne, propyne, butyne, pentyne, hexyne, heptyne, octyne, nonyne, and decyne. To indicate where the triple bond(s) is/are placed, number the carbons from one end of the molecule and use the lowest numbered carbon having the double bond as part of the compound name. Indicate multiple triple bonds with prefixes (di, tri, tetra, etc; the same prefixes used in inorganic compound names). Note: Alkanes, alkenes, and alkynes are collectively known as hydrocarbons. It is also possible to form cyclic chains of carbon atoms. One cyclic compound containing all single-bonded carbons has the same molecular formula as an alkene with the same number of carbons; in other words, one ring is the same as one double bond when it comes to molecular formulas. Ringcontaining compounds with only single-bonded carbons are called cycloalkanes; with doublebonded carbons, cycloalkenes. Note: the smallest possible cycloalkane contains three carbons. Only molecules with eight or more carbons in a ring can contain a triple bond. Halogens (F, Cl, Br, I) can replace hydrogens to form haloalkanes, haloalkenes, and haloalkynes. Note: there is an even exchange of one halogen for one hydrogen. Name halohydrocarbons as substituted hydrocarbon by indicating carbon attachment point for halogen. Use lowest possible carbon number for the parent chain attachment point for halogen. example: CH3CH(Br)CH2CH2CH2CH3 is 2-bromohexane Alcohols: molecules whose constituent elements are carbon, hydrogen, and oxygen with the condition that one hydrogen is attached to the oxygen. Alcohols are named after their parent alkanes with the same number of carbons, except that instead of the name ending with "-ane," it ends with "-anol." Therefore, have: methanol, ethanol, propanol, butanol, pentanol, hexanol, heptanol, octanol, nonanol, and decanol. General Formula: CnH2n+2O where n = 1, 2, 3 etc To indicate where the OH group(s) is/are placed, number the carbons from one end of the molecule and use the lowest numbered carbon having the OH bond as part of the compound name. Indicate multiple OH bonds with prefixes (di, tri, tetra, etc; the same prefixes used in inorganic compound names). examples: CH3CH(OH)CH2CH2CH2CH3 is 2-hexanol, not 5-hexanol CH2(OH)CH2CH2CH(OH)CH2CH2CH2CH2CH3 is 1,4-nonanediol Ethers: molecules whose constituent elements are carbon, hydrogen, and oxygen with the condition that the oxygen is bonded to two carbons. There are different ways to name ethers, which makes it too complicated to discuss here. AP students are not required to be able to name ethers. Note: It is impossible to have an ether containing only one carbon. General Formula: CnH2n+2O where n = 2, 3, 4 etc. Note: Alcohols and ethers have the same molecular formulas (except for C1 alcohol). Amines: molecules whose constituent elements are carbon, hydrogen, and nitrogen with the condition that the nitrogen is bonded to three carbons, or two carbons plus one hydrogen, or one carbon plus two hydrogens. Amines are named after their parent alkanes with the same number of carbons, except that instead of the name ending with "-ane," it ends with "-amine." Therefore, have: methaneamine, ethaneamine, propaneamine, butaneamine, pentaneamine, hexaneamine, heptaneamine, octaneamine, nonaneamine, and decaneamine. General Formula: CnH2n+3N where n = 1, 2, 3 etc To indicate where the N group(s) is/are placed, number the carbons from one end of the molecule and use the lowest numbered carbon having the N bond as part of the compound name. Indicate multiple N bonds with prefixes (di, tri, tetra, etc; the same prefixes used in inorganic compound names). Note: amines have one more hydrogen than the parent alkane because nitrogen uses an odd number of bonds. examples: CH3CH(NH2)CH2CH2CH2CH3 is 2-hexanamine, not 5-hexanamine CH2(NH2)CH2CH2CH(NH2)CH2CH2CH3 is 1,4-heptanediamine Aldehydes: molecules whose constituent elements are carbon, hydrogen, and oxygen with the condition that the oxygen is double bonded to a carbon, and the carbon also bonds to one hydrogen. General Formula: CnH2nO where n = 1, 2, 3 etc Aldehydes are named after their parent alkanes with the same number of carbons, except that instead of the name ending with "-ane," it ends with "-al." Therefore, have: methanal, ethanal, propanal, butanal, pentanal, hexanal, heptanal, octanal, nonanal, and decanal. Necessarily, the aldehyde carbon must be at the end of the carbon chain, so don't need to indicate attachment at C-1. example: CH3CH2CH2CH2CH2CHO is hexanal, not 1-hexanal Ketones: molecules whose constituent elements are carbon, hydrogen, and oxygen with the condition that the oxygen is double bonded to a carbon, and the carbon also bonds to two other carbons. General Formula: CnH2nO where n = 3, 4, 5 etc Ketones are named after their parent alkanes with the same number of carbons, except that instead of the name ending with "-ane," it ends with "-one." Note: Smallest possible ketone must have three carbons. Therefore, have: propanone, butanone, pentanone, hexanone, heptanone, octanone, nonanone, and decanone. Note: The ketone carbon cannot be at the end of the carbon chain, so must indicate attachment with lowest possible carbon number of chain. Aldehydes and ketones have the same general formula, so identity depends entirely on whether the oxygen is bonded to an end carbon or not. example: CH3CH2CH2CH2C(=O)CH3 is 2-hexanone, not 5-hexanone Carboxylic Acids: molecules whose constituent elements are carbon, hydrogen, and oxygen with the condition that one oxygen is double bonded to an end-chain carbon, that same carbon also single bonds to the other oxygen, which is also bonded to a hydrogen. General Formula: CnH2nO2 where n = 1, 2, 3 etc Acids are named after their parent alkanes with the same number of carbons, except that instead of the name ending with "-ane," it ends with "-oic acid." Therefore, have: methanoic acid, ethanoic acid, propanoic acid, butanoic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, and decanoic acid. Necessarily, the carboxylic acid carbon must be at the end of the carbon chain, so don't need to indicate attachment at C-1. example: CH3CH2CH2CH2CH2C(=O)OH is hexanoic acid, not 1-hexanoic acid Esters: molecules whose constituent elements are carbon, hydrogen, and oxygen with the condition that one oxygen is double bonded to an end-chain carbon, that same carbon also single bonds to the other oxygen, which is also bonded to another carbon (plus hydrogens attached to this carbon). General Formula: CnH2nO2 where n = 2, 3, 4 etc Esters are named after their parent carboxylic acids with the same number of carbons. However, have to indicate the identity of the carbon group to which the single-bonded oxygen is also connected. This portion is named first, and that portion of the name is taken from the parent alkane name by dropping the "ane" ending and replacing it with "yl" (for example: methyl, ethyl, propyl, etc.). Instead of the name ending with "-oic acid," it ends with "-oate." Note: Smallest possible ester must have two carbons. Therefore, have: "something" methanoate, "something" ethanoate, "something" propanoate, "something" butanoate, "something" pentanoate, "something" hexanoate, "something" heptanoate, "something" octanoate, "something" nonanoate, and "something" decanoate. Necessarily, the ester carbon must be at the end of the carbon chain, so don't need to indicate attachment at C-1. example: CH3CH2CH2CH2CH2C(=O)OCH3 is methyl hexanoate Amides: molecules whose constituent elements are carbon, hydrogen, oxygen, and nitrogen with the condition that the oxygen is double bonded to an end-chain carbon, and that same carbon single bonds to the nitrogen (which is also bonded to either two hydrogens, one hydrogen and one carbon, or two other carbons). General Formula: CnH2n +1NO where n = 1, 2, 3 etc Amides are named after their parent carboxylic acids with the same number of carbons. However, have to indicate the identity of the carbon group(s), if any, to which the nitrogen is also connected. This portion is named first using the same rule as given above for esters. Instead of the name ending with "-oic acid," it ends with "-amide." Therefore, have: "something" methanamide, "something" ethanamide, "something" propanamide, "something" butanamide, "something" pentanamide, "something" hexanamide, "something" heptanamide, "something" octanamide, "something" nonanamide, and "something" decanamide. Necessarily, the amide carbon must be at the end of the carbon chain, so don't need to indicate attachment at C-1. example: CH3CH2CH2CH2CH2C(=O)NHCH3 is N-methyl hexanamide Organic Reactions There are only a few organic reactions an AP chemistry student must know (that I recall seeing on an AP exam). (1) Combustion: usually of alkanes, but it can be any compound containing carbon, hydrogen, and oxygen only. Cannot be a compound containing nitrogen or halogens. reaction: CnH2n+2 + O2 → CO2 + H2O (unbalanced) (2) Hydrogenation: usually conversion of alkenes or alkynes to alkanes, but it can also be aldehydes and ketones to alcohols. reaction: CnH2n + H2 → CnH2n+2 or CnH2n-2 + H2 → CnH2n+2 (unbalanced) (3) Polymerization: formation of long carbon chains formed from repeating units. On an AP exam, students are typically asked to determine the monomer unit (the smallest unit that is repeated in the larger chain). There are two types of polymers: (a) addition polymers, and (b) condensation polymers. Addition polymers are formed from alkenes by adding one such chain to another to form a polyalkane chain. The monomer is the alkene. reaction: m[CnH2n + CnH2n] → -[CH2CH2CH2CH2]m- (chain grows in both directions) Condensation polymers are formed from (1) carboxylic acids and alcohols, or (b) carboxylic acids and amines. The first forms polyesters; the second forms polyamides. They are both produced by the removal of water, hence "condensation," meaning formation of H2O (l). The monomers are the combination of one molecule of each starting material joined together. reaction: m[RC(=O)OH + R*OH] → [RC(=O)OR*]m + H2O (R, R* = any carbon chain) m[RC(=O)OH + R*NH2] → [RC(=O)NHR*]m + H2O