Test Three
advertisement
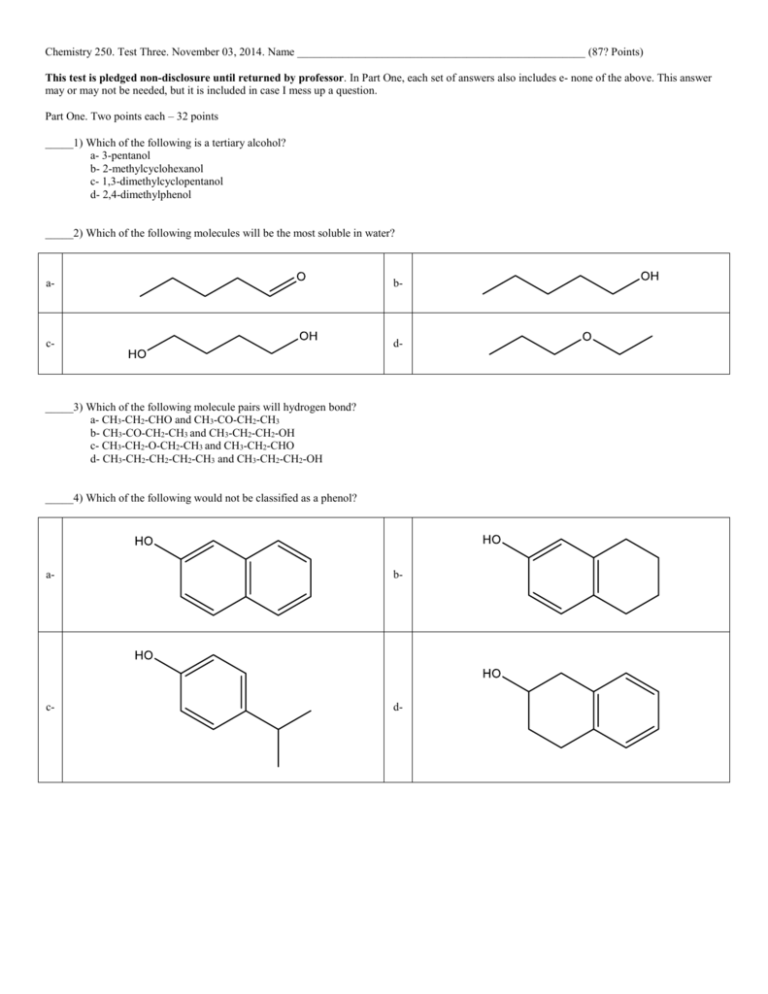
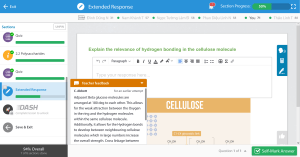
Chemistry 250. Test Three. November 03, 2014. Name ___________________________________________________ (87? Points) This test is pledged non-disclosure until returned by professor. In Part One, each set of answers also includes e- none of the above. This answer may or may not be needed, but it is included in case I mess up a question. Part One. Two points each – 32 points _____1) Which of the following is a tertiary alcohol? a- 3-pentanol b- 2-methylcyclohexanol c- 1,3-dimethylcyclopentanol d- 2,4-dimethylphenol _____2) Which of the following molecules will be the most soluble in water? a- b- c- d- _____3) Which of the following molecule pairs will hydrogen bond? a- CH3-CH2-CHO and CH3-CO-CH2-CH3 b- CH3-CO-CH2-CH3 and CH3-CH2-CH2-OH c- CH3-CH2-O-CH2-CH3 and CH3-CH2-CHO d- CH3-CH2-CH2-CH2-CH3 and CH3-CH2-CH2-OH _____4) Which of the following would not be classified as a phenol? a- b- c- d- _____5) Which of the following molecules is acetone? a- b- c- d- _____6) Which of the following molecules is acetylene? a- b- c- d- _____7) Which of the following molecules is not involved in hydrogen bonding? a- 1-butanethiol b- cyclohexanone c- diethyl ether d- 2-bromo-1-pentanol _____8) Which of the following reagents would not be used in an oxidation reaction? a- O2 b- FeO43c- H+, H2O d- ClO_____9) Which of the following groups is the most oxidized state? a- alcohol b- ketone c- aldehyde d- acid _____10) Which of the following reagents would not be used in a reduction reaction? a- NaOH b- LiAlH4 c- Sn, H+ d- H2, Pt _____11) Which of the following groups is the most reduced state? a- CH3-CH3 b- CH2=CH2 c- CH≡CH d- CH3-CH2-OH _____12) Which is the main product from the nitration of phenol? a- b- c- d- _____13) Which of the following was used by Lister as an antiseptic and disinfectant known as carbolic acid. a- b- c- d- Part Two. 1) (20 points) Nomenclature a- b- c- d- e- f- g- h- i- j- 2) (20 points) For each of the following reactions give the organic products. If more than one product is formed, circle the major product. 1) CH3-CH2-MgBr a- → + 2) H+ - b- + HBr → c- + H+, → d- + 1) Na → 2) CH3CH2Br e- + Cr2O72H+ → 3) (15 points) Definitions as we used them Hydrogen bonding Oxidation Reduction Carbonyl group Heterocyclic compound