organicclasswork2012answers
advertisement

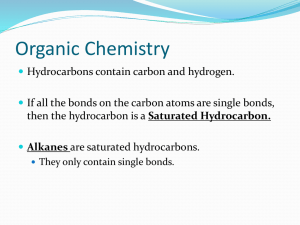
Organic Introduction Practice Questions 1. What are the bond angles associated with an end carbon? H H H C C H 0 1200 Why? 3 atoms are bonded to the this carbon atom which does not have non-bonding pairs of electrons surrounding it therefore the bond angle asscociated with the carbon would be 120o with a trigonal planar shape C O O H H 2. What are the bond angles associated with the central carbon in nail polish remover and why? _____0 Why? 4 atoms are bonded to the this carbon atom which does not have non-bonding pairs of electrons surrounding it therefore the bond angle associated with the carbon would be 109.5oand the shape would be tetrahedral 3. Which of the following is the general formula of an alkene? a) CnHn + 2 b) CnH2n + 2 c) CnH2n d) CnH2n-1 e) CnH2n-2 4. Which of the following is the general formula of an alkane? a) CnHn + 2 b) CnH2n + 2 c) CnH2n d) CnH2n-1 e) CnH2n-2 5. Why are alkanes considered to be saturated? Why are alkynes considered to be unsaturated? Alkanes are single bonded structures that maximize the number of hydrogen atoms in the molecule. 6. What is the name of each of the following hydrocarbons? a) C C C C b) C C C C C C C C C C C C C Octane C 4-ethyl-3,5-dimethyl-heptane C C c) C C C C C C C C C C C C 3,4,4,6-tetramethyl-nonane 7. Fill in the hydrogens and name the compound cis 6-methyl-3-octene or cis 3-methyl-5-octene H H H H H H H H a) H C C C C C C C C H H H H H H HCH H Fill in the carbons and the hydrogens and name the compound cyclopentene H H H H b) H H H H Name the compound 2-methyl-7-octyne f) C C C C C C C or 7-methyl-1-octyne C C 8. What are the molecular formulas of this structures? Draw all the atoms found in these structures? H H C H C C C H C H C H 9. a) Circle the longest chain in the following molecule/Redraw the molecule illustrating the longest chain b) What is the molecular formula of the following hydrocarbon? C16 H34 C) Name the molecule twice. d) Which of the formula would be the accepted name and why? C C C C C C C C C C C C C C C C C C C C C C–C–C–C- C–C–C–C–C–C C 4,7-diethyl-5,7-dimethyl-decane 4,7-diethyl-4,6-dimethyl-decane 10. Draw the carbon atoms and the line drawing for the the following hydrocarbons? a. 3 – ethyl – 2 –methyl – hexane b. 3,3 diethyl – heptane c. 2 – pentyne d. 2,3,4 trimethyl pentane e. 2,2 - dimethyl – 4- hexene 13. a) Draw three structural isomers of nonane b) name each nonane 3-methyl-octane 2,2-dimethyl-heptane 14. Draw the structure of cis 2-methyl - 2 – pentene including all carbons and hydrogens. 15. Draw the structure of trans 2 – methyl – 2 – pentene including all carbons and hydrogens. 16. What is the name of the following? trans-2-hexene H H H H H H C–C–C–C=C–C H H H H H H 17. What type of isomers are the following molecules of each other? How do they differ? What is the difference between L glucose and D glucose? Mirror Image isomer