Meilen, 18.11.2007 Organic chemistry practical course (OCPI) HS
advertisement

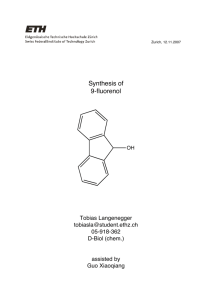
Meilen, 18.11.2007 Organic chemistry practical course (OCPI) HS 07 529-0229-00 Fabienne Vukotic 06-910-541 D-BIOL Assistant: Guo Xiaoqiang Synthesis of 9-Fluorenol 1. Method The method for the synthesis of 9-Fluorenol is a reduction of 9-Fluorenone with NaBH4. is an electrophilic aromatic substitution reaction: A Friedl-Crafts alkylation of o-xylene with a tert-butyl group from tert-butyl chloride with Iron (III) chloride – a Lewis acid – as catalyst. 2. Reaction Equation NaBH 4 O OH 3. Mechanism NaBH4 is chemoselective for keto groups: H O H Na Na B H NaBH4 O 1 eq. O H H B O H H B H H H 4 eq. can hydrate three more keto groups 3 H2 O, H B(OH)3 + OH B O 4 4 eq. 4. Physical and Chemical Properties of Substances 9-fluorenone CAS: 486-25-9 M: 180.21 g/mol d: 0.9 g/mL bp: 342 °C mp: 80-83 °C Safety Phrases -S22: Do not breathe dust -S24/25: Avoid contact with skin and eyes Methanol CAS: 67-56-1 M: 32.04 g/mol d: 0.791 g/mL bp: 64.7 °C mp: -98 °C n20D: 1.3288 MAK: 260 mg/m3, 200 mL/m3 CH-toxicity class: 3 Risk Phrases -R11: Highly Flammable -R23/24/25: Toxic by inhalation, in contact with skin and if swallowed -R39/23/24/25: Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed Safety Phrases -S7: Keep container tightly closed -S16: Keep away from sources of ignition - No smoking -S36/37: Wear suitable protective clothing and gloves -S45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) Sodium borohydride CAS: 16940-66-2 M: 37.83 g/mol d: 0.4 g/mL CH-toxicity class: 3 mp: 500 °C Risk Phrases -R15: Contact with water liberates extremely flammable gases -R24/25: Toxic in contact with skin and if swallowed -R34: Causes burns NaBH4 Safety Phrases -S22: Do not breathe dust -S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice -S36/37/39: Wear suitable protective clothing, gloves and eye/face protection -S43: In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. Never use water) -S45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) Sulfuric acid (25%) CAS: 7664-93-9 M: 98.08 g/mol d: 1.18 g/mL bp: 103 °C Risk Phrases -R35: Causes severe burns Safety Phrases -S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice -S30: Never add water to this product -S45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) 9-fluorenol CAS: 1689-64-1 M: 182.22 g/mol mp153-154 °C Safety Phrases -S24/25: Avoid contact with skin and eyes 5. Experimental Setup setup for reduction vacuum filtration safety bottle 100 100 mL 75 50 500 mL LaboBib© 300 50 AN AN AUS AUS 1500 100 o 0 U/min 250 500 C 200 150 1000 750 vacuum reflux: 20o 20o NS 14 20o 250 mL LaboBib© 300 50 AN AN AUS AUS 1500 0 U/min 250 500 100 o C 200 150 1000 750 6. Amounts of Substance used Substance measured amount [ml] [g] [mmol] eq 9-Fluorenone 0.60 3.3 2.5 Methanol 8.5 6.7 210 159 NaBH4 0.0498 1.32 1.0 H2SO4 (3M) 2.0 6.0 4.5 9-Fluorenone is the limiting reactant. NaBH4 is excess because it can not always hydrate 4 keto groups since they are very large groups. 7. Experimental Section (Procedure) 0.60 g of 9-Fluorenone is added to 6.0 mL methanol and heated until all 9fluorenone is dissolved. The solution is now yellow. After it has cooled down to RT, 0.0498 g NaBH4 is added and dissolved. It is allowed to stand at RT for 20min and is swirled occasionally. The solution turns to colorless again. 2.0 mL H2SO4 is added and a white precipitate forms. The contents in the flask are heated below the reflux point, 2.5 mL of methanol is added in 0.5 mL portions until a solution is obtained. The solution is allowed to cool to RT again and then is put in an ice bath for 10min. The substance is vacuum filtrated and washed thoroughly with distilled water until the filtrate is neutral. The substance is air-dried over night. The solid substance is now recrystallized and therefore put in a 50 mL twoneck round bottom flask and 0.8 mL distilled water and 5.3 mL methanol is added (a ratio of 1:5 would be optimal). It is heated to boiling under reflux and is then allowed to cool down. The product is vacuum filtrated and washed with distilled water. It is dried in the hot oven for 12h and then characterized. 8. Yield The theoretical yield is 3.33 mmol. The experimental yield is 0.349 g = 1.92 mmol. The yield percentage therefore is 57.5% 9. Characterization Melting point A melting point range of 154.0 – 154.9 °C was measured. The literature value for 9-fluorenol is 153-154 °C. IR-Spectrum (see attachement) bands [cm-1] 3298 ~3030 1607 1450 interpretation O-H unsat. C-H aromatic ring Literature spectrum http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi 10. Remarks and Discussion The melting point is a little bit too high. The IR spectrum is not very good and it shows impurities. 11. Literature Coblentz Society, Inc., "Evaluated Infrared Reference Spectra" in NIST Chemistry WebBook, NIST Standard Reference Database Number 69, Eds. P.J. Linstrom and W.G. Mallard, June 2005, National Institute of Standards and Technology, Gaithersburg MD, 20899. NIST Chemistry Webbook. 27. Oct. 2007 <http://webbook.nist.gov>. EMolecules - Searching the World's Chemistry. 2007. EMolecules, Inc. 28 Oct. 2007 <http://www.emolecules.com>. IGS Giftliste. 31 July 2007. BAG. 28 Oct. 2007 <http://igs.naz.ch/igs/igsServer/de.kisters.igs.igs40.servlets.igs40Servlet>. Kirste, Burkhard. "Chemikalien: Sicherheitsdaten." Institut für Chemie und Biochemie an der FU Berlin. 28 July 1994. FU Berlin. 27 Oct. 2007 <http://www.chemie.fu-berlin.de/chemistry/safety/chemsafety.html>. "Risk & Safety." Sigma-Aldrich. 2007. Sigma-Aldrich Co. 27 Oct. 2007 <http://www.sigmaaldrich.com/Help_Pages/Help_Welcome/Product_ Search/Risk___Safety_Statements.html#Safety%20Phrases>. Sigma-Aldrich. 2007. Sigma-Aldrich Co. 27 Oct. 2007 <http://www.sigmaaldrich.com/Area_of_Interest/Europe_Home/ Switzerland__Schweiz_.html>.