Carbohydrates Monosaccharides: Aldoses and Ketoses
advertisement

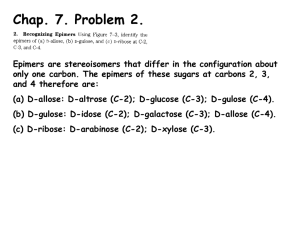
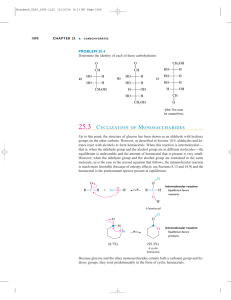
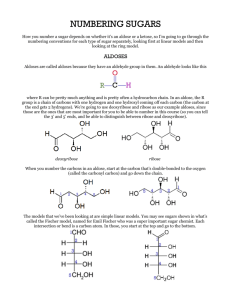
Carbohydrates Molecules with formula CnH2nOn. Fisher projections: ≡ ≡ D-glucose (C6H12O6) D-/L- notation: After placing most-oxidized carbon on top, sugars with last chiral –OH on right are D-sugars. Monosaccharides: Aldoses and Ketoses Aldoses are polyhydroxy-aldehydes. D-glyceraldehyde D-erythrose D-arabinose D-glucose (C3H6O3) (C4H8O4) (C5H10O5) (C6H12O6) an aldotriose an aldotetrose an aldopentose an aldohexose Monosaccharides: Aldoses and Ketoses Ketoses are polyhydroxy-ketones. dihydroxyacetone (C3H6O3) D-erythrulose D-ribulose D-fructose (C4H8O4) (C5H10O5) (C6H12O6) a ketotriose a ketotetrose a ketopentose a ketohexose Epimers Epimers differ in stereochemistry at one carbon. 1 6 D-mannose the C2-epimer of glucose D-glucose D-galactose the C4-epimer of glucose Monosaccharides Equilibrate Between Acyclic and Cyclic Forms rotate t t C4-C5 bond ≡ D-glucose cyclic hemiacetals stereoisomers, “anomers” Equilibrating Glucose Anomers Haworth projections p j Timescale of reaction: mins-hrs < 1% 32% -D-glucopyranose -anomer 64% -D-glucopyranose -anomer The Anomeric Effect -D-glucopyranose 32% 64% -D-glucopyranose “mutarotation” Advantage: The anomeric effect. Advantage: Hydroxyl group is equatorial. + Interaction between Osp3 lone pair and C1-O * antibonding orbital is stabilizing, t bili i and d orbitals bit l are oriented correctly. Anomeric effect not possible for -anomer; orbitals don’t line up. Ketoses Also Equilibrate Between Acyclic and Cyclic Forms ≡ D-fructose cyclic h ik t l hemiketals Also forms pyranose (6 (6-membered) b d) rings. i -D-fructofuranose 70% 30% -D-fructofuranose , but found most often in complex sugars. Glycosides catalytic H+ R –OH -D-glucose + Timescale of reaction: decades R R -glycoside -glycoside ((often f the major product) Glycosides do not undergo mutarotation. Disaccharides via Enzymatic Glycosidation H HO glycosidase -glycosidase D-glucose CH2OH (+ H2O) O H H HO H OH H O HO HO CH H 2 HOH C 2 O sucrose + D-fructose H OH H Enzyme catalyst increases reaction rate, makes k reaction ti stereospecific. t ifi In absence of catalyst, at pH 7, reaction too slow to observe.