CARBOHYDRATES
advertisement

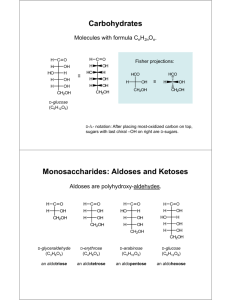
CARBOHYDRATES Carbohydrate Classifications: Monosaccharides - sweeteners; building blocks of larger carbohydrates. Simple carbohydrates - cannot be hydrolyzed. dihydroxyacetone; D-glyceride; D-glucose; D-galactose; D-fructose Individual saccharides can be joined by glycosidic linkages. Prefix indicates how many saccharides are linked together — di-, tri-, tetra-, penta-, hexa-, heptasaccharide... Complex carbohydrates - can be hydrolyzed into smaller carbohydrates. maltose; cellobiose; lactose; sucrose Polysaccharides - more than 10 saccharides. starch (amylose, amylopectin, glycogen); cellulose Saccharide - polyhydroxyaldehyde or polyhydroxyketone with the formula (CH2O)n where n = 3, 4, 5, ... -ose: suffix used in most monosaccharide names (i.e. glucose) and designations (see below). Aldose or Ketose: indicates that the saccharide is an aldehyde or a ketone, respectively. Tri-, tetr-, pent-, hex-, or heptose: indicates the number of carbons in a saccharide. These designations can be combined with aldo or keto listed first. Examples: aldotriose; ketoheptose. D or L configuration of a saccharide is determined by the position of the oxygen on the last chiral center. Left is L; Right is D. For cyclic saccharides look for the CH2OH: Up is D; Down is L. (See examples on Glucose page.) Cyclic Saccharides - formed when a hydroxyl group reacts at the carbonyl carbon pyranose: cyclic monosaccharide having a six atom ring furanose: cyclic monosaccharide having a five atom ring anomeric carbon: the carbon bonded to two oxygens in the structures below. α or β configuration of cyclic saccharides is determined by the position of the oxygen on the anomeric carbon: Up is β; Down is α. (See examples on Glucose page.) Hemiacetal: can be recognized by the presence of an OH on the anomeric carbon. Ring readily reopens. Acetal: can be recognized by the absence of an OH on the anomeric carbon. Ring “locked” closed. hemiacetal acetal CH 2OH O OH HO anomeric carbon OH OH R stands for a carbon CH 2OH or a carbon chain O OR HO anomeric carbon OH OH Reducing Sugar - a saccharide that will reduce Benedict's solution (oxidizing the saccharide). This includes cyclic hemiacetals of saccharides as well as the open chain forms of saccharides on the following pages. Acetals do not with Benedict’s solution.