Chapter 20 Carbohydrates
advertisement

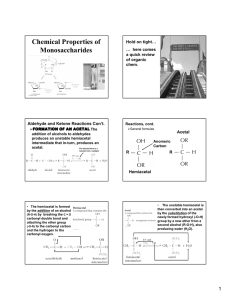
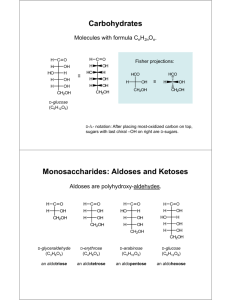
Chapter 20 Carbohydrates Carbohydrates Carbohydrate: A polyhydroxyaldehyde or polyhydroxyketone, or a substance that gives these compounds on hydrolysis. Monosaccharide: A carbohydrate that cannot be hydrolyzed to a simpler carbohydrate. • Monosaccharides have the general formula CnH2nOn, where n varies from 3 to 8. • Aldose: A monosaccharide containing an aldehyde group. • Ketose: A monosaccharide containing a ketone group. Monosaccharides The suffix -ose indicates that a molecule is a carbohydrate. The prefixes tri-, tetra, penta, and so forth indicate the number of carbon atoms in the chain. Those containing an aldehyde group are classified as aldoses. Those containing a ketone group are classified as ketoses. There are only two trioses: Monosaccharides There are only two trioses: ◦ Often aldo- and keto- are omitted and these compounds are referred to simply as trioses. ◦ Although “triose” does not tell the nature of the carbonyl group, it at least tells the number of carbons. Monosaccharide Monosaccharides with ◦ three carbons: trioses ◦ Five carbons: pentose ◦ Six carbons: hexose ◦ And so on … Monosacharides Figure 12.1 Glyceraldehyde, the simplest aldose, contains one stereocenter and exists as a pair of enantiomers. Enantiomers Enantiomers: a molecule has a nonsuperimposable mirror image ◦ Chiral molecule – has four different groups Monosaccharides Fischer projection: A two-dimensional representation for showing the configuration of tetrahedral stereocenters. • Horizontal lines represent bonds projecting forward from the stereocenter. • Vertical lines represent bonds projecting to the rear. • Only the stereocenter is in the plane. Monosacharides In 1891, Emil Fischer made the arbitrary assignments of D- and Lto the enantiomers of glyceraldehyde. • D-monosaccharide: the –OH is attached to the bottom-most assymetric center (the carbon that is second from the bottom) is on the right in a Fischer projection. Monosacharides • L-monosaccharide: the -OH is on the left in a Fischer projection. D,L-Monosaccharides • The most common D-tetroses and D-pentoses are: D,L-Monosaccharides The three most common D-hexoses are: Amino Sugars Amino sugars contain an -NH2 group in place of an -OH group. • Only three amino sugars are common in nature: D-glucosamine, D-mannosamine, and D-galactosamine. N-acetyl-D-glucosamine is an acetylated derivative of D-glucosamine. Cyclic Structure • Aldehydes and ketones react with alcohols to form hemiacetals • Cyclic hemiacetals form readily when the hydroxyl and carbonyl groups are part of the same molecule and their interaction can form a five- or six-membered ring. Epimers Diastereomers that differ in configuration at only on asymmetric center Table 20-1 p532 Table 20-2 p532 Examples Draw Fisher projections for all 2-ketopentoses. Which are D2-ketopentoses, which are L-2-ketopentoses? Prefer to table 12.2 (your textbook) to write their names Haworth Projections • Figure 12.2 D-Glucose forms these two cyclic hemiacetals. Haworth Projections • A five- or six-membered cyclic hemiacetal is represented as a planar ring, lying roughly perpendicular to the plane of the paper. • Groups bonded to the carbons of the ring then lie either above or below the plane of the ring. • The new carbon stereocenter created in forming the cyclic structure is called the anomeric carbon. • Stereoisomers that differ in configuration only at the anomeric carbon are called anomers. • The anomeric carbon of an aldose is C-1; that of the most common ketose is C-2. Haworth Projections In the terminology of carbohydrate chemistry, ◦ b means that the -OH on the anomeric carbon is on the same side of the ring as the terminal -CH2OH. ◦ a means that the -OH on the anomeric carbon is on the side of the ring opposite from the terminal -CH2OH. ◦ A six-membered hemiacetal ring is called a pyranose, and a fivemembered hemiacetal ring is called a furanose because these ring sizes correspond to the heterocyclic compounds furan and pyran. Haworth Projections ◦ Aldopentoses also form cyclic hemiacetals. ◦ The most prevalent forms of D-ribose and other pentoses in the biological world are furanoses. ◦ The prefix “deoxy” means “without oxygen.” at C2 Haworth Projections D-Fructose (a 2-ketohexose) also forms a five-membered cyclic hemiacetal. Examples Give structure of the cyclic hemiacetal formed by ◦ 4-hydroxybutanal ◦ 5-hydroxypentanal Chair Conformations • For pyranoses, the six-membered ring is more accurately represented as a strain-free chair conformation. Chair Conformations • In both Haworth projections and chair conformations, the orientations of groups on carbons 1- 5 of b-D-glucopyranose are up, down, up, down, and up. Chair Conformations Examples Which OH groups are in the axial position in β-D-mannopyranose β-D-idopyranose Mutarotation Mutarotation: The change in specific rotation that accompanies the equilibration of a- and b-anomers in aqueous solution. ◦ Example: When either a-D-glucose or b-D-glucose is dissolved in water, the specific rotation of the solution gradually changes to an equilibrium value of +52.7°, which corresponds to 64% beta and 36% alpha forms. Formation of Glycosides • Treatment of a monosaccharide, all of which exist almost exclusively in cyclic hemiacetal forms, with an alcohol gives an acetal. Formation of Glycosides • A cyclic acetal derived from a monosaccharide is called a glycoside. • The bond from the anomeric carbon to the -OR group is called a glycosidic bond. • Mutarotation is not possible for a glycoside because an acetal, unlike a hemiacetal, is not in equilibrium with the open-chain carbonyl-containing compound. Formation of Glycosides • Glycosides are stable in water and aqueous base, but like other acetals, are hydrolyzed in aqueous acid to an alcohol and a monosaccharide. • Glycosides are named by listing the alkyl or aryl group bonded to oxygen followed by the name of the carbohydrate in which the ending -e is replaced by ide. Examples Draw a Haworth projection and a chair conformation for methyl -D-mannopyranoside. Label the anomeric carbon and glycosidic bond