Chapter 21
Chapter 21
Carbohydrates
Carbohydrates
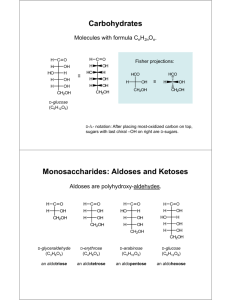
They have the molecular formulas C n
(H
2
O) m
Compounds that can be hydrolyzed to polyhydroxy aldehydes or polyhydroxy ketones are also classified as carbohydrates
Simple Carbohydrates Are Monosacharides
Complex carbohydrates contain two or more sugar units linked together
•disaccharides
•oligosaccharides
•polysaccharides
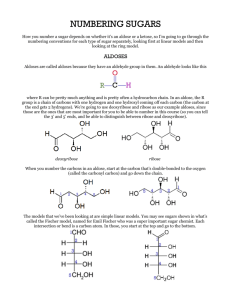
Polyhydroxy aldehydes are aldoses
Polyhydroxy ketones are ketoses
D and L notations are used to describe the configurations of carbohydrates
1
6
2
3
4
5
D-(+)-Glyceraldehyde L-(–)-Glyceraldehyde
Figure 6.18
Configurations of Aldoses
Aldotetroses have two stereogenic centers and four stereoisomers
Diastereomers that differ in configuration at only one stereogenic carbon are called epimers
A ketose has one less stereogenic center than aldoses, therefore they have fewer stereoisomers
Redox Reactions of Monosaccharides
The carbonyl of aldoses and ketoses can be reduced by the carbonyl-group reducing agents
Oxidation
The aldehyde groups can be oxidized by Br
2
Ketones and alcohols cannot be oxidized by Br
2
In a basic solution, ketoses are converted into aldoses
A strong oxidizing agent such as HNO
3 aldehyde and the alcohol groups can oxidize the
Osazone Formation
Aldoses and ketoses react with three equivalents of phenylhydrazine
The C-2 epimers of aldoses form identical osazones
Reaction of Ketoses with Phenylhydrazine
The carbon chain of an aldose can be increased by one carbon in a Kiliani–Fischer synthesis
The Ruff degradation shortens an aldose chain by one carbon
Preparation of the Calcium D-Gluconate for the
Ruff Degradation
Cyclic Structure of Monosaccharides
Hemiacetal Formation anomer anomer
The specific rotation of pure α -D-glucose or β -D-glucose changes over time to reach an equilibrium (mutarotation)
Figure 27.2
Note …
• If an aldose can form a five- or six-membered ring, it will exist predominantly as a cyclic hemiacetal
• Six-membered rings are called pyranoses
• Five-membered rings are called furanoses
• A sugar with an aldehyde, a ketone, a hemiacetal, or a hemiacetal group is a reducing sugar
The structures of cyclic sugars are best represented by the Haworth projections
Haworth projections allow us to see the relative orientation of the OH groups in the ring
Ketoses also exist predominantly in cyclic forms
β -D-Glucose Is More Stable
β -D-glucose is the predominant form at equilibrium
Acylation of Monosaccharides
The OH groups of monosaccharides show the chemistry of typical alcohols
Alkylation of the OH Groups
Formation of Glycosides
The acetal (or ketal) of a sugar is called a glycoside
Mechanism of Glycoside Formation
Formation of an N -Glycoside
The Anomeric Effect
The formation of a glycoside favors the α -glucoside product: the anomeric effect
Determination of Ring Size
Approach 1
The size of the ring can be determined from the structure of the open-chain form
Determination of Ring Size
Approach 2
An acetal of the monosaccharide is oxidized with excess
HIO
4
The α -hydroxyaldehyde formed from HIO
4 oxidation is further oxidized to formic acid and another aldehyde
Disaccharides
Composed of two monosaccharide subunits hooked together by an acetal linkage
In α -maltose, the OH group bonded to the anomeric carbon is axial
Maltose is a reducing sugar
In cellobiose, the two subunits are hooked together by a
β -1,4’-glycosidic linkage
Cellobiose is a reducing sugar
In lactose, the two different subunits are joined by a
β -1,4’-glycosidic linkage
Lactose is a reducing sugar
The most common disaccharide is sucrose
Sucrose is not a reducing sugar
Polysaccharides
Amylose is a component of starch
Structure of Chitin
Structure of Cellulose
Intramolecular hydrogen bonding
Amylopectin is another polysaccharide component of starch that has a branched structure
Structure of amylose
The αααα -1,4-glycosidic linkages in amylose cause this polymer to form a left-handed helix.
An example of a naturally occurring product derived from carbohydrates
Blood type is determined by the nature of the sugar bound to the protein on the surface of red blood cells