chapter 4
advertisement

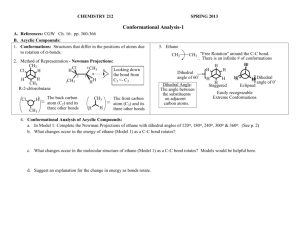
CHEM 3013 ORGANIC CHEMISTRY I LECTURE NOTES CHAPTER 4 1. Conformations of n-alkanes Single bonds allow rotation around the sigma bond axis. The atoms attached to the bonds will try to achieve a conformation (position of atoms in space due to bond rotation) where the local interaction of all groups is minimized. By studying a few simple alkanes, we can derive some "guidepost numbers" that represent some destabilizations which occur in common molecular arrangements. These numbers can then be applied in more complex settings to evaluate the most probable conformation of a molecule. This will be of great importance in understanding the effect of structure on chemical reactivity. a. Ethane A 3-D perspective drawing such as the saw horse is sometimes written as a side view projection of convenience. The vertical lines in each side view projection are in the plane of the paper. Hashed lines drawn to the left are behind the plane. Bold lines drawn to the right are out in front of the plane. A more useful representation to show rotation around a C-C bond is the Newman projection. The view is down the C-C bond axis with one carbon eclipsing the other. Bonds attached to the front carbon are shown by lines intersecting at the center of a circle. Bonds attached to the rear carbon are shown by lines attached to the circumference of the circle. H H H H H HH H H H H H H H H H HCCH dihedral angle = 0 (rear H's behind front H's) Staggered Conformation H H H HCCH dihedral angle = 60 (rear H's bisect front H,s) H H H E HH Newman Projection H H E N E R G Y H H H H Eclipsed Conformation H Sideview Projection Sawhorse Representation H HH H H E E E 2.9 Kcal/Mole S 0 60 S 120 180 Rotational Angle Conformations of ethane S 240 300 360 1 b. 2 Butane Butane has a more complex conformational equilibrium than ethane. The fully eclipsed form of butane 1 is 4.5 Kcal/mole more destabilized (higher in energy) than the anti form 4. Since we know the value of two H,H eclipsing interactions from our analysis of ethane, i.e. 2 x 1 Kcal/mole = 2 Kcal/mole, we can now assign an energy to the CH3, CH 3 eclipsing interaction of 2.5 Kcal/mole. Similarly, the value of a CH 3, H eclipsing interaction can be determined as (3.81)/2 = 1.4 Kcal/mole. A final interaction that can be obtained from this study is the value of gauche butane destabilization of 0.9Kcal/mole. CH3 H 3C H H CH3 H HH H H CH3 H H CH3 H H H3CCH3 H H H HH Fully Eclipsed 1 H3CH H H H H Gauche 2 H CH3 H CH 3 H Partly Eclipsed 3 CH3 H H CH3 Anti 4 1 1 3 3 E N E R G Y H H H CH3 CH3 CH3 H H H CH3 4.5 Kcal/mole 3.8 Kcal/mole 2 2 0.9 Kcal/mole 0 60 4 120 180 Rotational Angle 240 300 360 Conformations of n-butane 2. Conformations of Cyclohexanes Von Baeyer predicted in 1885 that the stability of cycloalkanes would be related to their angle strain and should be correlated to the internal bond angles of the planar structures. The further away from the ideal tetrahedral angle of 109˚, the more strain the structure should have. 3 60 90 108 120 128.6 135 Bond Angles for Flat Cycloalkanes Isomers can be compared by determining their Heats of Formation. Members of a homologous series can be compared by evaluation of their heats of formation per -CH2- unit. By doing this we can see that von Baeyer's prediction is in error, cyclopentane is not the most stable cycloalkane, cyclohexane is. Moreover, this compound is strain free, it has the same heat of formation per -CH2- unit as does n-hexane. Von Baeyer's mistaken assumption was that all these molecules would be planar. Heat of Formation for Acyclic (linear) -CH2Ring Size 3 4 5 6 7 8 Heats of Formation in Kcal/mole +12.7 + 6.8 - 18.4 - 29.5 - 28.2 - 29.7 Heats of Formation in Kcal/mole per CH2 unit + 4.2 + 1.7 - 3.7 - 4.9 - 4.0 - 3.7 = -4.90 Kcal/mole Total Strain Energy in Kcal/mole + 27 + 26 + 6 0 + 6 + 10 CH2 Strain Energy in Kcal/mole + 9.1 + 6.6 + 1.2 0 + 0.9 + 1.2 Heats of Formation of Cycloalkanes a. Cyclopropane and Cyclobutane Cyclopropane has no conformational mobility, and is thus forced to be planar. The six pairs of eclipsing hydrogens in cyclopropane contribute 6.0 Kcal/mole of Torsional Energy . The balance of the 27 Kcal/mole of Total Strain Energy is contributed by Angle Strain . Cyclobutane has almost as much Total Strain as does cyclopropane because its greater number of ring hydrogens (8 vs. 6) results in more Torsional Strain even though it has less Angle Strain. In addition, evidence shows the cyclobutane is not quite flat. It has enough conformational mobility that the ring can bend slightly, so that one carbon lies about 25˚ above the plane of the other three. 4 HH H H H H CH2 H H 6 Pairs of Eclipsing H's H H 6 x 1 = 6.0 Kcal/mole Torsional Strain Total Strain - Torsional Strain = Angle Strain + 27 Kcal/mole - 6.0Kcal/mole = 21 Kcal/mole Angle Strain Strain in Cyclopropane H H H H Not Quite Eclipsed 25deg. H2C CH2 Bent Conformation of Cyclobutane b. Cyclopentane Von Baeyer predicted cyclopentane to be strain free (CCC angles of 108˚),but experiments indicate that it has 6 Kcal/mole of strain energy. Although cyclopentane has no Angle Strain it does have considerable Torsional Strain due to the ten pairs of neighboring hydrogens. Cyclopentane has enough conformational mobility to adopt a puckered shape in order to minimize destabilization. In the envelope conformation, four of the ring carbons are in approximately the same plane, with one carbon bent out of the plane. Look down C1-C2 axis H 1 H CH2 2 2 H 1 H CH2 CH2 Planar cyclopentane - Predicted Torsional Strain - 10 Kcal/mole Actual Total Strain - 6 Kcal/mole Cyclopentane deviates from planarity to relieve Torsional Strain Envelope Conformation of Cyclopentane 3. 5 Conformations of Cyclohexane Cyclohexane has no Strain Energy, that is it has no Angle Strain nor Torsional Strain. Obviously, this precludes the possibility of cyclohexane existing in the planer structure (12 pairs of eclipsing H's would result in at least 12 Kcal/mole of Torsional Energy). Cyclohexane has enough flexibility that it can adopt a strain free structure known as the Chair conformation. In this structure the bond angles are all close to tetrahedral and all pairs of hydrogens are completely staggered with respect to one another. The latter point can be seen by looking down each carboncarbon bond in turn to produce the Newman projection shown below. H H H H H H HH H H H H H H H H H H Planar Conformation H H H H H Chair Conformation CCC angles 120 deg. At least 12 Kcal/mole Torsional Strain H H CCC angles 109 deg. No Torsional Strain H H H H2 C C H2 H H H H Chair Conformation of Cyclohexane a. Axial - Equatorial Interconversion The chair conformation has two distinct types of hydrogen atoms. These different hydrogens are named the Axial and Equatorial hydrogens as shown below. The axial hydrogens are perpendicular to the mean ring plane, whereas the equatorial hydrogens project out along the ring circumference (i.e. equator). The molecular axis is a three-fold axis, rotation by 120˚ about the axis leaves the molecular representation unchanged. 6 Six Equivalent Axial H's Six Equivalent Equatorial Hydrogens Three-fold axis of symmetry Axial and Equatorial Hydrogens The chair conformation of cyclohexane has two structurally different types of hydrogen atoms (axial and equatorial), but attempts to discern any difference in type by chemical reactions are unsuccessful. Usually, different "types" of hydrogens demonstrate (often subtle) differences in chemical behavior. However, all hydrogens in cyclohexane behave identically. Similarly, we might expect to find two isomeric forms of a monosubstituted cyclohexane (substituent at the axial or equatorial position). This is not the case. Cyclohexane is a dynamic structure. A concerted partial rotation about the carbon-carbon bonds changes one chair conformation to another in which the axial and equatorial bonds have changed places. The interconversion of chair conformations, usually referred to as a ring-flip, is shown below. Ring Flip move this carbon up move this carbon down Interconversion of Chair Conformations There are other, higher energy, cyclohexane conformations called the boat, twist-boat and half-chair forms. 7 Flagpole Boat - Four pairs of eclipsing hydrogens, in addition to steric repulsions of "flagpole" hydrogens Twist Boat - The two "flagpole" hydrogens are offset so that they do not directly run into each other. Half Chair- The four planar carbons result in Eclipsing interactions Conformations of Cyclohexane half-chair 1 10.1 Kcal E N E R G Y half-chair 2 boat twist-boat 7.0 Kcal 5.5 Kcal chair 1 chair 2 Energetics of Cyclohexane Forms 4 Monosubstituted Cyclohexanes The interconversion of equatorial monosubstituted cyclohexane to its alternative chair form generates a conformation which has the substituent axial. This form is no longer identical to the equatorial form from which it arose. These two forms have differing energy content due to the different environments of the substituent. H H H H H H H H CH3 H H H H H H CH3 H Axial-Equatorial Methylcyclohexane H H H H H H H 8 1,3-Diaxial interaction, steric repulsion, 1.8 Kcal/mole Similar to Guache butane worth 1.8 Kcal/mole H H H H H H H H HH 2 3C C C H2 H CH3 H H H H2 H C C H2 H H CH3 CH3 H No Destabilizing interactions 1,3-Diaxial Destabilization The amount of energy required to convert from an equatorial to an axial form is called the A-Value for a given substituent. Typical A Values for Monosubstituted Cyclohexanes (Kcal/mol) F Cl, Br OH OCH3 NH2 0.25 0.50 0.70 0.70 1.80 CH3 C2H5 n-C3H7 i-C3H7 t-C4H9 1.80 1.90 2.10 2.10 5.40 Typical A-Values for Monosubstituted Cyclohexanes 5. Disubstituted Cyclohexanes a. Trans 1,2-Dimethylcyclohexane This stereoisomer of dimethylcyclohexane has two substituents on opposite sides of the cyclohexane ring in the chair conformation. This gives rise to two possible chair conformers: one with both methyls equatorial and one with both axial. They have different energies as a result of different destabilizing interactions present. The diequatorial conformer is lower in energy than the diaxial conformer by 2.7 Kcal/mole. The diequatorial form will account for 99% of the equilibrium mixture; the diaxial for only 1%. CH3 CH3 CH3 CH3 Chair 2 Chair 1 H H H H H2 H C C H2 H CH3 CH3 H The diequatorial conformation has one butane gauche type interaction...results in 0.9 Kcal/mol destabilization energy H H H H2 CH3 C H C H2 H CH3 The diaxial conformation has two 1,3-diaxial type interactions...3.6 Kcal/mol destabilization energy The difference in energy between the two forms favors the diequatorial conformer by 2.7 Kcal/mol. Keq = e-∆G/RT Keq = 0.01 Interconversion of trans-1,2dimethylcyclohexane b. cis-1,2-Dimethylcyclohexane This stereoisomer of dimethylcyclohexane has two substituents on the same side of the cyclohexane ring in the chair conformation. The two possible conformations each have one substituent axial and one substituent equatorial. Thus they have the same extent of destabilizing interactions. 9 10 CH3 CH3 CH3 CH3 Chair 2 Chair 1 H H H H2 CH3 C CH3 H C H2 H H H H2 H C H H H CH3 C H2 CH 3 H Same for this form. The monoaxial-monoequatorial form has one gauche type interaction and one 1,3-diaxial type interaction for 2.7 Kcal/mole destabilization energy There is no difference in energy between the two forms... each will be present in equal amounts (50%:50%). Interconversion of cis-1,2dimethylcyclohexane c. cis-1,3-dimethylcyclohexane This compound has two substituents on the same side of the ring and has two possible chair conformations. One conformation has a new type of 1,3-diaxial interaction...a 1,3-diaxial methyl-methyl interaction. This interaction is worth 3.7 Kcal/mol of destabilizing energy. CH3 CH3 Chair 2 Chair 1 H H H H H2 H C CH3 H CH3 H H H CH3H NO destabilizing steric interactions H2 H C H H H CH3CH3 Two gauche butane type interactions and one 1,3-diaxial methyl-methyl interaction for a total destabilization energy of 5.5 Kcal/mol. Interactions in cis-1,3-dimethylcyclohexane 11