The Double Y Diagram
advertisement
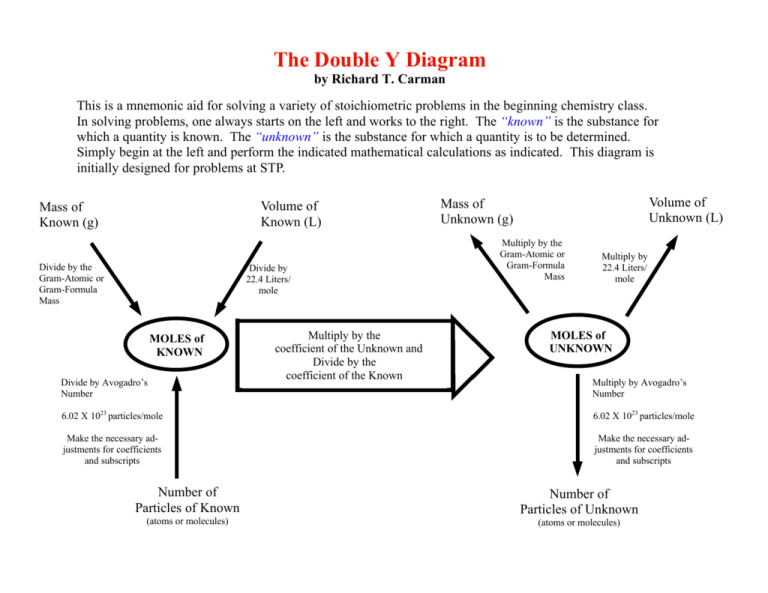
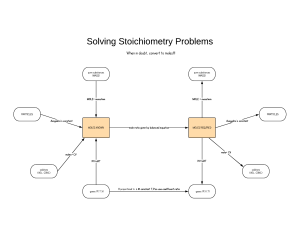
The Double Y Diagram by Richard T. Carman This is a mnemonic aid for solving a variety of stoichiometric problems in the beginning chemistry class. In solving problems, one always starts on the left and works to the right. The “known” is the substance for which a quantity is known. The “unknown” is the substance for which a quantity is to be determined. Simply begin at the left and perform the indicated mathematical calculations as indicated. This diagram is initially designed for problems at STP. Volume of Known (L) Mass of Known (g) Divide by the Gram-Atomic or Gram-Formula Mass Divide by 22.4 Liters/ mole MOLES of KNOWN Divide by Avogadro’s Number Multiply by the coefficient of the Unknown and Divide by the coefficient of the Known Volume of Unknown (L) Mass of Unknown (g) Multiply by the Gram-Atomic or Gram-Formula Mass Multiply by 22.4 Liters/ mole MOLES of UNKNOWN Multiply by Avogadro’s Number 6.02 X 1023 particles/mole 6.02 X 1023 particles/mole Make the necessary adjustments for coefficients and subscripts Make the necessary adjustments for coefficients and subscripts Number of Particles of Known Number of Particles of Unknown (atoms or molecules) (atoms or molecules)