Mole Worksheet #1: moles ↔ particles
advertisement

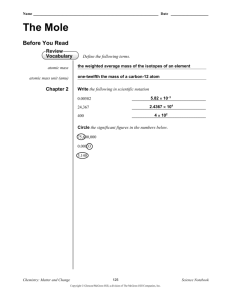
Name ________________________ Date ______________ Period _____ Mole Worksheet #1: moles ↔ particles The mole is simply a number equal to 602,000,000,000,000,000,000,000 or 6.02 x 1023. How large is a mole?? Well, if you had a mole of pennies, you would have enough money to pay all the expenses of the United States for the next billion years. A mole of large marshmallows would cover the United States to a depth of more than 600 miles. Questions 1. A mole is “counting number”. The following are also counting numbers. What number do each of the following terms represent? dozen_________________ gross_______________ pair________________________ mole________________ score ______________ ream (of paper)_______________ 2. List the four types of “representative particles” that are referred to in your textbook? Converting moles to representative particles: 6.02 X 1023 representative particles 1 mole Converting representative particles to moles: representative particles = moles X moles = representative particles X 6.02 X 10 23 1 mole representative particles 3. Determine the number of representative particles in each of the following. Show factor label method. A. 0.250 mol silver B. 8.56 x 10-3 mol NaCl C. 35.4 mol CO2 D. 0.425 mol N2 4. Determine the number of moles in each of the following. Show factor label method. A. 3.25 x 1020 atoms Pb B. 4.96 x 1024 molecules glucose C. 1.56 x 1023 formula units NaOH D. 1.25 x 1025 Cu2+ ions 5. Make the following conversions. Show factor label method. A. 1.51 x 1015 atoms Si to mol Si B. 4.25 x 10-2 mol NO2 to molecules NO2 C. 8.95 x 1025 molecules CCl4 to mol CCl4 D. 5.90 mol Ca to atoms Ca