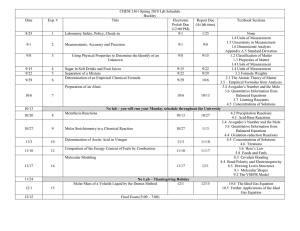
Solving Stoichiometry Problems Flowchart
advertisement
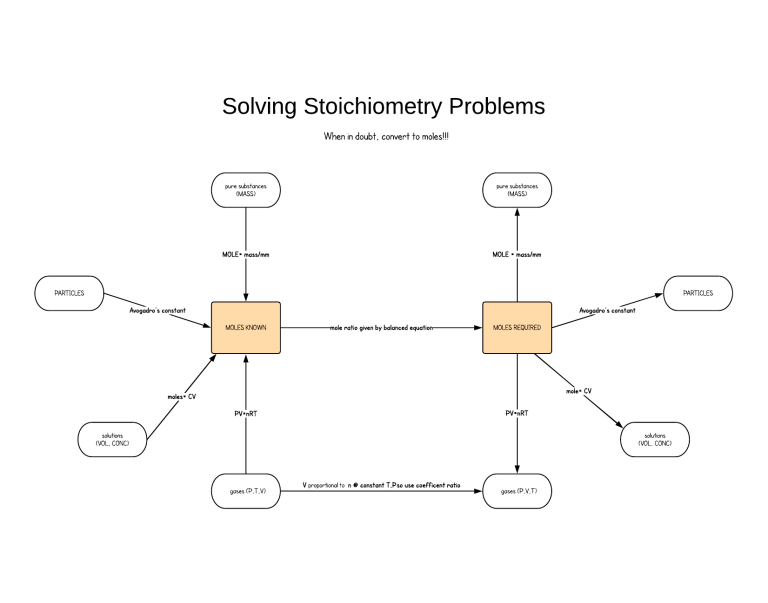
Solving Stoichiometry Problems Whenindoubt, convert tomoles!!! pure substances (MASS) puresubstances (MASS) MOLE= mass/mm MOLE = mass/mm PARTICLES PARTICLES Avogadro's constant Avogadro's constant MOLESKNOWN mole ratio given by balanced equation MOLESREQUIRED mole= CV moles= CV PV=nRT PV=nRT solutions (VOL, CONC) solutions (VOL, CONC) gases (P,T,V) V proportional to n @constant T,Pso use coeff icent ratio gases (P,V,T)