Chapter 9: Phase Diagrams
advertisement

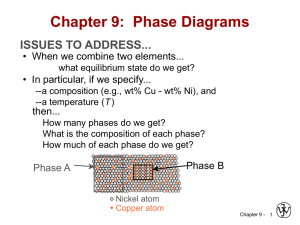
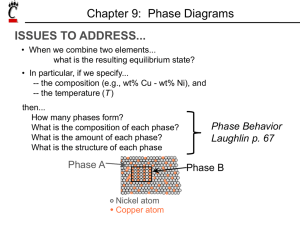
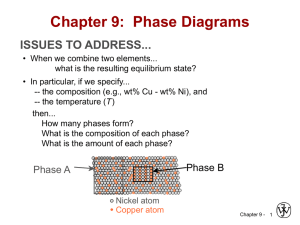
Chapter 9: Phase Diagrams ISSUES TO ADDRESS... • When we combine two elements... what equilibrium state do we get? • In particular, if we specify... --a composition (e.g., wt% Cu - wt% Ni), and --a temperature (T ) then... How many phases do we get? What is the composition of each phase? How much of each phase do we get? Phase B Phase A Nickel atom Copper atom Chapter 9 - 1 Phase Equilibria: Solubility Limit Introduction – Solutions – solid solutions, single phase – Mixtures – more than one phase Adapted from Fig. 9.1, Callister 7e. Sucrose/Water Phase Diagram • Solubility Limit: Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar. Temperature (°C) Max concentration for which only a single phase solution occurs. 100 Solubility Limit 80 L (liquid) 60 L 40 (liquid solution i.e., syrup) 20 + S (solid sugar) Pure Water Pure Sugar If Co < 65 wt% sugar: syrup 0 20 40 6065 80 100 If Co > 65 wt% sugar: syrup + sugar. Co =Composition (wt% sugar) Chapter 9 - 2 Components and Phases • Components: The elements or compounds which are present in the mixture (e.g., Al and Cu) • Phases: The physically and chemically distinct material regions that result (e.g., α and β). AluminumCopper Alloy β (lighter phase) α (darker phase) Adapted from chapter-opening photograph, Chapter 9, Callister 3e. A phase maybe defined as a homogeneous portion of a system that has Chapter 9 - 3 uniform physical and chemical characteristics. Effect of T & Composition (Co) • Changing T can change # of phases: path A to B. • Changing Co can change # of phases: path B to D. B (100°C,70) D (100°C,90) 1 phase watersugar system Adapted from Fig. 9.1, Callister 7e. Temperature (°C) 100 2 phases L 80 (liquid) 60 L (liquid solution 40 i.e., syrup) + S (solid sugar) A (20°C,70) 20 2 phases 0 0 20 40 60 70 80 100 Co =Composition (wt% sugar) Chapter 9 - 4 Phase Equilibrium Equilibrium: minimum energy state for a given T, P, and composition (i.e. equilibrium state will persist indefinitely for a fixed T, P and composition). Phase Equilibrium: If there is more than 1 phase present, phase characteristics will stay constant over time. Phase diagrams tell us about equilibrium phases as a function of T, P and composition (here, we’ll always keep P constant for simplicity). Chapter 9 - 5 Unary Systems Triple point Chapter 9 - 6 Phase Equilibria Simple solution system (e.g., Ni-Cu solution) Crystal Structure electroneg r (nm) Ni FCC 1.9 0.1246 Cu FCC 1.8 0.1278 • Both have the same crystal structure (FCC) and have similar electronegativities and atomic radii (W. Hume – Rothery rules) suggesting high mutual solubility. • Ni and Cu are totally miscible in all proportions. Chapter 9 - 7 Unary Systems Single component system Consider 2 metals: Cu has melting T = 1085oC Ni has melting T = 1453oC (at standard P = 1 atm) T T liquid liquid 1085oC 1453oC solid solid Cu Ni Chapter 9 - 8 What happens when Cu and Ni are mixed? Binary Isomorphous Systems 2 components Complete liquid and solid solubility Expect Tm of solution to lie in between Tm of two pure components T T liquid liquid L 1453oC 1085oC solid 0 Cu S wt% Ni solid 100 Ni For a pure component, complete melting occurs before T increases (sharp phase transition). But for multicomponent systems, there is usually a coexistence of L and S. Chapter 9 - 9 Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course: -binary systems: just 2 components. -independent variables: T and Co (P = 1 atm is almost always used). T(°C) • Phase Diagram for Cu-Ni system • 2 phases: 1600 1500 L (liquid) α (FCC solid solution) L (liquid) 1400 liq 1300 L + αus lid so 1200 α (FCC solid solution) 1100 1000 us d i u 0 20 40 60 80 • 3 phase fields: L L+α α Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys, P. Nash (Ed.), ASM International, Materials Park, OH (1991). 100 wt% Ni Chapter 9 - 10 Phase Diagrams: # and types of phases • Rule 1: If we know T and Co, then we know: --the # and types of phases present. A(1100°C, 60): 1 phase: α B(1250°C, 35): 2 phases: L + α 1600 L (liquid) 1500 B (1250°C,35) • Examples: T(°C) 1400 1300 solution) 1100 1000 +α α L (FCC solid 1200 Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys, P. Nash (Ed.), ASM International, Materials Park, OH, 1991). Cu-Ni phase diagram s u d ui liq us d i l so A(1100°C,60) 0 20 40 60 80 100 wt% Ni Chapter 9 - 11 Phase Diagrams: composition of phases • Rule 2: If we know T and Co, then we know: --the composition of each phase. • Examples: T(°C) Cu-Ni system A TA Co = 35 wt% Ni tie line dus ui q i l 1300 L (liquid) At T A = 1320°C: +α L Only Liquid (L) B s idu l T o B s CL = Co ( = 35 wt% Ni) α α At T D = 1190°C: L+ (solid) 1200 D Only Solid ( α) TD Cα = Co ( = 35 wt% Ni) 20 3032 35 4043 50 At T B = 1250°C: CLCo Cα wt% Ni Both α and L Adapted from Fig. 9.3(b), Callister 7e. 9.3(b) is adapted from Phase Diagrams CL = C liquidus ( = 32 wt% Ni here) (Fig. of Binary Nickel Alloys, P. Nash (Ed.), ASM Cα = C solidus ( = 43 wt% Ni here) International, Materials Park, OH, 1991.) Chapter 9 - 12 Determining phase composition in 2-phase region: 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cα amount of Ni (42.5 wt% Ni). Chapter 9 - 13 Phase Diagrams: weight fractions of phases • Rule 3: If we know T and Co, then we know: --the amount of each phase (given in wt%). • Examples: Co = 35 wt% Ni At T A : Only Liquid (L) W L = 100 wt%, W α = 0 At T D: Only Solid ( α) W L = 0, Wα = 100 wt% At T B : Both α and L WL = Wα = 43 − 35 S = = 73 wt % R + S 43 − 32 R = 27 wt% R +S Cu-Ni system T(°C) A TA L (liquid) 1300 tie line dus i l i qu B R S TB L 1200 TD 20 +α D 3032 35 CLCo α + L id s ol us α (solid) 40 43 50 Cα wt% Ni Adapted from Fig. 9.3(b), Callister 7e. (Fig. 9.3(b) is adapted from Phase Diagrams of Binary Nickel Alloys, P. Nash (Ed.), ASM International, Materials Park, OH, 1991.) Chapter 9 - 14 Lever Rule: Derivation Since we have only 2 phases: WL + Wα = 1 (1) Conservation of mass requires that: Amount of Ni in α-phase + amount of Ni in liquid phase = total amount of Ni or (2) Wα Cα + WL C L = Co From 1st condition, we have: Sub-in to (2): Wα = 1 − WL (1 − WL )Cα + WL C L = Co Solving for WL and Wα gives : WL = Cα − Co Cα − C L Wα = Co − C L Cα − C L Chapter 9 - 15 The Lever Rule • Tie line – connects the phases in equilibrium with each other - essentially an isotherm T(°C) 1300 tie line dus i l i qu L (liquid) 1200 L id s ol S R 30C C 40 C α L o wt% Ni WL = us Mα ML α (solid) +α R 20 α + L B TB How much of each phase? Think of it as a lever (teeter-totter) 50 S M α ⋅S = M L ⋅R Adapted from Fig. 9.3(b), Callister 7e. ML S C − C0 = = α M L + M α R + S C α − CL Wα = R C − CL = 0 R + S Cα − CL Chapter 9 - 16 Ex: Cooling in a Cu-Ni Binary • Phase diagram: Cu-Ni system. • System is: --binary i.e., 2 components: Cu and Ni. T(°C) L (liquid) 130 0 L: 35 wt% Ni α: 46 wt% Ni i.e., complete solubility of one component in another; α phase field extends from 0 to 100 wt% Ni. • Consider Co = 35 wt%Ni. A 32 --isomorphous L: 35wt%Ni 35 B C α 46 43 D 24 1200 L+ Cu-Ni system L: 32 wt% Ni 36 α + L α: 43 wt% Ni E L: 24 wt% Ni α: 36 wt% Ni α (solid) 1100 20 30 Adapted from Fig. 9.4, Callister 7e. 35 Co 40 50 wt% Ni Chapter 9 - 17 Microstructures in Isomorphous Alloys Microstructures will vary on the cooling rate (i.e. processing conditions) 1. Equilibrium Cooling: Very slow cooling to allow phase equilibrium to be maintained during the cooling process. a (T>1260oC): start as homogeneous liquid solution. b (T ~ 1260oC): liquidus line reached. α phase begins to nucleate. Cα = 46 wt% Ni; CL = 35 wt% Ni c (T= 1250oC): calculate composition and mass fraction of each phase. Cα = 43 wt% Ni; CL = 32 wt% Ni d (T~ 1220oC): solidus line reached. Nearly complete solidification. Cα = 35 wt% Ni; CL = 24 wt% Ni e (T<1220oC): homogeneous solid solution with 35 wt% Ni. Chapter 9 - 18 Non-equilibrium cooling Since diffusion rate is especially low in solids, consider case where: Cooling rate >> diffusion rate in solid Cooling rate << diffusion rate in liquid (equilibrium maintained in liquids phase) Chapter 9 - 19 Non-equilibrium cooling a’ (T>1260oC): start as homogeneous liquid solution. b’ (T ~ 1260oC): liquidus line reached. α phase begins to nucleate. Cα = 46 wt% Ni; CL = 35 wt% Ni c’ (T= 1250oC): solids that formed at pt b’ remain with same composition (46wt%) and new solids with 42 wt% Ni form around the existing solids (Why around them?). d’ (T~ 1220oC): solidus line reached. Nearly complete solidification. •Previously solidified regions maintain original composition and further solidification occurs at 35 wt% Ni. e (T<1220oC): Non-equilibrium solidification complete (with phase segregation). Chapter 9 - 20 Cored vs Equilibrium Phases • Cα changes as we solidify. • Cu-Ni case: First α to solidify has Cα = 46 wt% Ni. Last α to solidify has Cα = 35 wt% Ni. • Fast rate of cooling: Cored structure • Slow rate of cooling: Equilibrium structure First α to solidify: 46 wt% Ni Last α to solidify: < 35 wt% Ni Uniform C α: 35 wt% Ni Cored structure can be eliminated by a homogenization heat treatment at a temperature below the solidus point Chapter 9 - 21 for the particular alloy composition. Mechanical Properties: Cu-Ni System • Effect of solid solution strengthening on: --Ductility (%EL,%AR) Tensile Strength (MPa) At RT 400 TS for pure Ni 300 TS for pure Cu 200 0 20 40 60 80 100 Cu Ni Composition, wt% Ni Adapted from Fig. 9.6(a), Callister 7e. --Peak as a function of Co Elongation (%EL) --Tensile strength (TS) 60 %EL for pure Cu %EL for pure Ni 50 40 30 20 0 20 Cu 40 60 80 100 Ni Composition, wt% Ni Adapted from Fig. 9.6(b), Callister 7e. --Min. as a function of Co Chapter 9 - 22 Binary-Eutectic Systems has a special composition with a min. melting T. 2 components Cu-Ag system T(°C) Ex.: Cu-Ag system 1200 • 3 single phase regions L (liquid) 1000 (L, α, β) α L + α 779°C • Limited solubility: L+β β 800 T α: mostly Cu 8.0 71.9 91.2 E β: mostly Ag 600 • TE : No liquid below TE α+β 400 • CE : Min. melting TE composition 200 0 • Eutectic transition L(CE) α(CαE) + β(CβE) 20 40 60 CE 80 100 Co , wt% Ag Adapted from Fig. 9.7, Callister 7e. Chapter 9 - 23 Eutectic Point Eutectic point: Where 2 liquidus lines meet (pt. E). Sometimes also referred to as invariant point. Eutectic Reaction: cool L(CE) α(CαE) + β(CβE) heat similar to one component (pure) system except 2 solid phases. Cu-Ag phase diagram Eutectic Isotherm: Horizontal solidus line at T = TE. Single phase regions: α, β, L-phase 2-Phase coexistence regions: α+β, α+L and β+L Chapter 9 - 24 EX: Pb-Sn Eutectic System (1) • For a 40 wt% Sn-60 wt% Pb alloy at 150°C, find... --the phases present: α + β T(°C) --compositions of phases: CO = 40 wt% Sn Cα = 11 wt% Sn Cβ = 99 wt% Sn --the relative amount of each phase: Wα = C - CO S = β R+S Cβ - Cα Pb-Sn system 300 200 L (liquid) α L+ α 18.3 150 100 59 99 - 40 = = 67 wt% 99 - 11 88 C - Cα Wβ = R = O Cβ - Cα R+S L+β β 183°C 61.9 R 97.8 S α+β = = 40 - 11 29 = = 33 wt% 99 - 11 88 0 11 20 Cα 40 Co 60 80 C, wt% Sn 99100 Cβ Adapted from Fig. 9.8, Callister 7e. Chapter 9 - 25 EX: Pb-Sn Eutectic System (2) • For a 40 wt% Sn-60 wt% Pb alloy at 200°C, find... --the phases present: α + L T(°C) --compositions of phases: CO = 40 wt% Sn Cα = 17 wt% Sn CL = 46 wt% Sn --the relative amount of each phase: CL - CO 46 - 40 = Wα = CL - Cα 46 - 17 6 = = 21 wt% 29 Pb-Sn system 300 L (liquid) L+α 220 200 α R L+β β S 183°C 100 CO - Cα 23 = WL = = 79 wt% CL - Cα 29 α+β 0 17 20 Cα 40 46 60 Co CL 80 C, wt% Sn Adapted from Fig. 9.8, Callister 7e. Chapter 9 - 26 100 Microstructures in Eutectic Systems: I • Co < 2 wt% Sn T(°C) Adapted from Fig. 9.11, Callister 7e. L: Co wt% Sn 400 L α 1. One component rich composition. a: start with homogeneous liquid. b: α-phase solids with liquid. Compositions and mass fractions can be found via tie lines and lever rule. c: α-phase solid solution only. Net result: polycrystalline α solid. Cooling at this composition is similar to binary isomorphous systems. L 300 α 200 L+ α (Pb-Sn System) α: Co wt% Sn TE 100 α+ β 0 Co 10 20 30 C , wt% Sn o 2 (room T solubility limit) Chapter 9 - 27 Microstructures in Eutectic Systems: II • 2 wt% Sn < Co < 18.3 wt% Sn Adapted from Fig. 9.12, Callister 7e. L: Co wt% Sn T(°C) 400 L 2. One-component rich but cooling to α + β coexistence. d: homogeneous liquid. e: α + L phase (same as previous but at different compositions and mass fractions). f: all α-phase solid solution. g: α + β phase (passing through solvus line leads to exceeding solubility limit and β phase precipitates out). Net result: polycrystalline α-solid with fine β crystals. L α 300 L+α α: Co wt% Sn α 200 TE α β 100 α+ β 0 10 20 Pb-Sn system 30 Co Co , wt% 2 (sol. limit at T room ) 18.3 (sol. limit at TE) Sn Chapter 9 - 28 Microstructures in Eutectic Systems: III • Co = CE • Result: Eutectic microstructure (lamellar structure) --alternating layers (lamellae) of α and β crystals. T(°C) L: Co wt% Sn 300 Micrograph of Pb-Sn eutectic microstructure L Pb-Sn system L+α α 200 L+β β 183°C TE 100 α+β 0 20 18.3 Adapted from Fig. 9.13, Callister 7e. 40 β: 97.8 wt% Sn α: 18.3 wt%Sn 60 CE 61.9 80 160 µm Adapted from Fig. 9.14, Callister 7e. 100 97.8 C, wt% Sn Chapter 9 - 29 Lamellar Eutectic Structure α β Sn α Pb rich L β Sn rich Pb In order to achieve large homogeneous regions, long diffusion lengths are required. Adapted from Figs. 9.14 & 9.15, Callister 7e. Lamellar structure forms because relatively short diffusion lengths Chapter 9 - 30 are required. Microstructures in Eutectic Systems: IV • 18.3 wt% Sn < Co < 61.9 wt% Sn • Result: α crystals and a eutectic microstructure L: Co wt% Sn T(°C) 300 L α L Pb-Sn system α L L+α α 200 R TE L+β β S S R primary α eutectic α eutectic β 0 20 18.3 Adapted from Fig. 9.16, Callister 7e. 40 60 61.9 Cα = 18.3 wt% Sn CL = 61.9 wt% Sn S Wα = = 50 wt% R+S WL = (1- Wα) = 50 wt% • Just below TE : α+β 100 • Just above TE : 80 Co, wt% Sn 100 97.8 Cα = 18.3 wt% Sn Cβ = 97.8 wt% Sn Wα= S = 73 wt% R+S Wβ = 27 wt% Chapter 9 - 31 Hypoeutectic & Hypereutectic (Pb-Sn System) 300 L T(°C) Adapted from Fig. 9.8, Callister 7e. (Fig. 9.8 adapted from Binary Phase Diagrams, 2nd ed., Vol. 3, T.B. Massalski (Editor-inChief), ASM International, Materials Park, OH, 1990.) α 200 L +α TE α+β 100 0 20 40 hypoeutectic: Co = 50 wt% Sn (Figs. 9.14 and 9.17 from Metals Handbook, 9th ed., Vol. 9, Metallography and Microstructures, American Society for Metals, Materials Park, OH, 1985.) α α α Microconstituent: an element of a microstructure with identifiable and L+β β characteristic structure (at pt. m there are 2 microconstituents: primary α and eutectic structures) 60 80 eutectic 61.9 100 hypereutectic: (illustration only) eutectic: Co = 61.9 wt% Sn β β α α β α 175 µm Adapted from Fig. 9.17, Callister 7e. Co, wt% Sn β β β 160 µm eutectic micro-constituent Adapted from Fig. 9.14, Callister 7e. Adapted from Fig. 9.17, Callister 7e. (Illustration only) Chapter 9 - 32 Intermediate phases Intermediate solid solutions (intermediate phases): Solid solutions that do not extend to pure components in the phase diagram. Cu-Zn Terminal solid solutions: α and η. Intermediate solid solutions: β, γ, δ and ε. Tie lines and lever rule can be used to determine compositions and wt% of phases. e.g. at 800oC with 70 wt% Zn CL = 78 wt% Zn Cγ = 67 wt% Zn Chapter 9 - 33 Intermetallic Compounds Adapted from Fig. 9.20, Callister 7e. Mg2Pb Note: intermetallic compound forms a discrete line - not an area - because stoichiometry (i.e. composition) is Chapter exact. 9 - 34 Eutectoid & Peritectic • Eutectic - liquid in equilibrium with two solids L cool α + β heat • Eutectoid - solid phase in equation with two solid phases intermetallic compound S2 S1+S3 - cementite γ cool α + Fe3C (727ºC) heat • Peritectic - liquid + solid 1 solid 2 (Fig 9.21) S1 + L S2 cool δ + L heat γ (1493ºC) Chapter 9 - 35 Eutectoid & Peritectic Cu-Zn Phase diagram Peritectic transition γ + L Adapted from Fig. 9.21, Callister 7e. Eutectoid transition δ γ+ε Chapter 9 - 36 δ Congruent phase transformation Congruent transformation: no change in composition upon phase transformation. (for example: allotropic transformation) Incongruent transformation: phase transformation where at least one of the phases go through composition change. (for example: isomorphous, eutectic and eutectoid system) Chapter 9 - 37 Ceramic phase diagrams Al2O3-Cr2O3 MgO-Al2O3 Chapter 9 - 38 Gibbs Phase Rule A criterion for the number of phases that will coexist within a system at equilibrium Number of nonNumber of compositional variables (Temperature & Pressure) phases present P+F=C+N Degree of freedom (externally controllable parameters: i.e. T, P, and C) Number of components e.g. Cu-Ag phase diagram Cu and Ag are the only components -> C = 2 Temperature is the only non-compositional variable here (i.e. fixed pressure). -> N = 1 (but in general N = 2) When 2 phases are present -> P = 2 which leads to F = C+N-P = 2+1-2 = 1 When only 1 phase is present. -> P = 1 which leads to F = 2 What does this mean? Why should you care? Chapter 9 - 39 Gibbs Phase Rule In the previous example of Cu-Ag phase diagram, when F = 1, only one parameter (T or C) needs to be specified to completely define the system. e.g. (for α+L region) If T is specified to be 1000oC, compositions are already determined (Cα and CL). Or If composition of the a phase is specified to be Cα then both T and CL are already determined. The nature of the phases is important, not the relative phase amounts. Cα CL Chapter 9 - 40 Gibbs Phase Rule When F = 2, both T and C have to be specified to completely define the state of the system. e.g.(for α region) If T is specified to be 800oC, Cα can be any where between 0 to ~8 wt% Ag) Or If composition of the a phase is specified to be Cα = 3 wt%, then T and can be any where between ~600 to 1100oC. Cα Chapter 9 - 41 Gibbs Phase Rule Where in the Cu—Ag diagram, is there a 0 degree of freedom? (i.e. T, P, and C are all fixed)---eutectic isotherm F=3-P=0 Chapter 9 - 42 Concept Check • Question: For a ternary system, three components are present; temperature is also a variable. What is the maximum number of phases that may be present for a ternary system, assuming that pressure is held constant? Chapter 9 - 43 Iron-Carbon Systemstructural material Ferrite Magnets TVR Tuscan Speed 6, high-performance sports car with an austempered ductile iron crankshaft. The world's first bridge made of iron in 1779. The entire structure is made of cast iron. (near Broseley, UK) Chapter 9 - 44 Iron-Carbon System Steel bridges The Akashi Kaikyo bridge, a 3-span 2hinged truss-stiffened suspension bridge. completed in 1998. It connects Kobe with Awaji Island. It is the world's longest suspension bridge, with a span between the towers of 1.9 km. Millau Viaduct in France, the highest bridge in the world. Golden Gate Bridge Chapter 9 - 45 Iron-Carbon System • Iron-carbon (Fe-C) system is one of the most important binary systems due to the versatile uses of the iron-based structural alloys. • This phase diagram is so important in understanding the equilibrium structure, and in the design of heat treatment process of iron alloys. • The most important part of the phase diagram is the region below6.7 w% carbon. All practical iron-carbon alloys contain C below 6.7 w%. This part is of the phase diagram is thus the most analyzed part of the iron carbon phase diagram. Chapter 9 - 46 Iron-Carbon System Typical metal (e.g. Cu) T Iron T(oC) Liquid Liquid 1538 Tm Ferrite (BCC) 1394 Austenite (FCC) Solid 912 Ferrite (BCC) Chapter 9 - 47 Iron-Carbon System Iron-Iron Carbide Diagram Chapter 9 - 48 Remarks on Fe-C system • C is an interstitial element in Fe matrix. • C has limited solid solubility in the alpha BCC phase (narrow region close to pure iron). Max solid solubility of C in alpha iron is 0.022 w% at 727 °C. • Alpha iron an be made magnetic below 768°C. • Austenite phase is not stable below at 727 °C. Max solid solubility of C into austenite is 2.14 w% at 1147°C, much larger that that in alpha phase. • Austenite is a non-magnetic phase, and heat treatment of Fe-C alloys involving austenite is so important. • Cementite phase (intermetallic, Fe3C) forms over a large region of the Fe-C phase diagram, but it is a metastable phase (heating above 650 °C for years decomposes this phase into alpha iron and graphite). Cementite is very hard and brittle. • δ ferrite is stable only at relatively high T, it is of no technological importance and is not discussed further. Chapter 9 - 49 Ferrite (90x) Relatively soft Density: 7.88g/cm3 Austenite (x325) Chapter 9 - 50 Classification Scheme of Ferrous Alloys • Iron Pure ion contains less than 0.008wt% C • Steel 0.008-2.14 wt% C, in practice,<1.0 wt% C • Cast Iron 2.14-6.70 wt% C, in practice, <4.5 wt% C Chapter 9 - 51 Iron-Carbon (Fe-C) Phase Diagram cool Eutectoid cooling: γ (0.76 wt% C) L ⇒ γ + Fe3C -Eutectoid (B): heat T(°C) 1600 δ L 1400 1200 γ ⇒ α + Fe3C γ +L γ (austenite) γ α+ 800 α 120 µm Result: Pearlite = alternating layers of α and Fe3C phases (Adapted from Fig. 9.27, Callister 7e.) γ +Fe3C 727°C = Teutectoid R 400 0 (Fe) S B S α+Fe3C 1 0.76 C eutectoid 600 L+Fe3C R γ γ γ γ 1000 A 1148°C 2 3 4 5 6 Fe3C (cementite) • 2 important points -Eutectic (A): α (0.022 wt% C) + Fe3C (6.7 wt% C) 6.7 4.30 Co, wt% C Fe3C (cementite-hard) α (ferrite-soft) Adapted from Fig. 9.24,Callister 7e. Chapter 9 - 52 Pearlite colonies Pearl-microscope picture (X500) Mechanically, pearlite has properties intermediate between the Chapter 9 soft, ductile ferrite and the hard, brittle cementite. 53 Hypoeutectoid Steel T(°C) 1600 δ L γ γ γ γ γ +L γ 1200 (austenite) γ γ γ γ 1000 α γ γ α γ αγ 800 γ + Fe3C r s 727°C αRS w α =s/(r +s) 600 w γ =(1- wα ) 400 0 (Fe) pearlite α + Fe3C 1 C0 2 3 4 5 0.76 α L+Fe3C 1148°C 6 Co , wt% C 100 µm pearlite Adapted from Figs. 9.24 and 9.29,Callister 7e. (Fig. 9.24 adapted from Binary Alloy Phase Diagrams, 2nd ed., Vol. 1, T.B. Massalski (Ed.-inChief), ASM International, Materials Park, OH, 1990.) 6.7 w pearlite = w γ w α =S/(R+S) w Fe3C =(1-w α ) (Fe-C System) Fe3C (cementite) 1400 Hypoeutectoid steel proeutectoid ferrite Adapted from Fig. 9.30,Callister 7e. Chapter 9 - 54 Hypereutectoid Steel T(°C) 1600 δ L γ γ γ +L γ 1200 (austenite) γ γ 1000 γ γ γ γ 800 Fe3C w Fe3C =r/(r +s) w γ =(1-w Fe3C ) r α R 600 400 0 (Fe) pearlite L+Fe3C 1148°C γ +Fe3C 0.76 γ γ γ γ (Fe-C System) s S α +Fe3C 1 Co w pearlite = w γ w α =S/(R+S) w Fe3C =(1-w α ) 2 3 4 5 6 Fe3C (cementite) 1400 Adapted from Figs. 9.24 and 9.32,Callister 7e. (Fig. 9.24 adapted from Binary Alloy Phase Diagrams, 2nd ed., Vol. 1, T.B. Massalski (Ed.-inChief), ASM International, Materials Park, OH, 1990.) 6.7 Co , wt%C 60 µmHypereutectoid steel pearlite proeutectoid Fe3C Adapted from Fig. 9.33,Callister 7e. Chapter 9 - 55 Example: Phase Equilibria For a 99.6 wt% Fe-0.40 wt% C at a temperature just below the eutectoid, determine the following a) composition of Fe3C and ferrite (α) b) the amount of carbide (cementite) in grams that forms per 100 g of steel c) the amount of pearlite and proeutectoid ferrite (α) Chapter 9 - 56 Chapter 9 – Phase Equilibria Solution: a) composition of Fe3C and ferrite (α) b) the amount of carbide (cementite) in grams that forms per 100 g of steel CO = 0.40 wt% C Cα = 0.022 wt% C CFe C = 6.70 wt% C 3 1600 δ 1200 0.4 − 0.022 = x 100 = 5.7g 6.7 − 0.022 L γ α = 94.3 g 1000 γ + Fe3C 727°C R S α + Fe3C 600 400 0 L+Fe3C 1148°C (austenite) 800 Fe3C = 5.7 g γ +L Fe3C (cementite) Fe3C Co − Cα 1400 = x100 T(°C) Fe3C + α CFe3C − Cα Cα CO 1 2 3 4 5 6 Co , wt% C 6.7 CFe 3C Chapter 9 - 57 Chapter 9 – Phase Equilibria c. the amount of pearlite and proeutectoid ferrite (α) note: amount of pearlite = amount of γ just above TE 1600 δ L 1400 T(°C) γ +L γ Co − Cα γ = x 100 = 51.2 g 1200 (austenite) γ + α Cγ − Cα 1000 γ + Fe3C 800 727°C RS pearlite = 51.2 g proeutectoid α = 48.8 g 600 400 0 L+Fe3C 1148°C α + Fe3C 1 Cα C Cγ O 2 3 4 5 6 Co , wt% C Chapter 9 - 58 Fe3C (cementite) Co = 0.40 wt% C Cα = 0.022 wt% C Cpearlite = Cγ = 0.76 wt% C 6.7 Effect of alloying elements on Fe-C phase diagram Chapter 9 - 59 Alloying Steel with More Elements Ti Mo Si W Cr Mn Ni wt. % of alloying elements Adapted from Fig. 9.34,Callister 7e. (Fig. 9.34 from Edgar C. Bain, Functions of the Alloying Elements in Steel, American Society for Metals, 1939, p. 127.) • Ceutectoid changes: C eutectoid (wt%C) T Eutectoid (°C) • Teutectoid changes: Ni Cr Si Ti Mo W Mn wt. % of alloying elements Adapted from Fig. 9.35,Callister 7e. (Fig. 9.35 from Edgar C. Bain, Functions of the Alloying Elements in Steel, American Society for Metals, 1939, p. 127.) Chapter 9 - 60 Summary • Phase diagrams are useful tools to determine: --the number and types of phases, --the wt% of each phase, --and the composition of each phase for a given T and composition of the system. • Alloying to produce a solid solution usually --increases the tensile strength (TS) --decreases the ductility. • Binary eutectics and binary eutectoids allow for a range of microstructures. Chapter 9 - 61