Chapter 9
advertisement

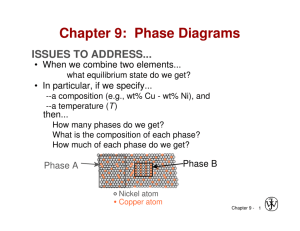
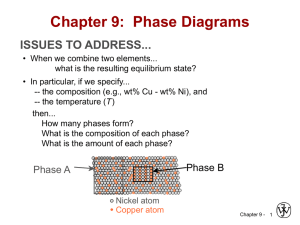
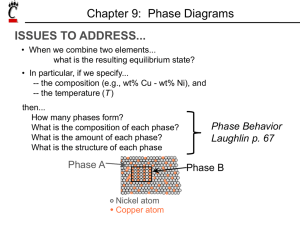
Chapter 9: Phase Diagrams ISSUES TO ADDRESS... • When we combine two elements... what equilibrium state do we get? • In particular, if we specify... --a composition (e.g., wt% Cu - wt% Ni), and --a temperature (T ) then... How many phases do we get? What is the composition of each phase? How much of each phase do we get? Phase B Phase A Nickel atom Copper atom Chapter 9 - 1 Phase Equilibria: Solubility Limit Introduction – Solutions – solid solutions, single phase – Mixtures – more than one phase Adapted from Fig. 9.1, Callister 7e. Sucrose/Water Phase Diagram • Solubility Limit: Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar. Temperature (°C) Max concentration for which only a single phase solution occurs. 100 Solubility Limit 80 L (liquid) 60 L 40 (liquid solution i.e., syrup) 20 + S (solid sugar) Pure Water Pure Sugar If Co < 65 wt% sugar: syrup 0 20 40 6065 80 100 If Co > 65 wt% sugar: syrup + sugar. Co =Composition (wt% sugar) Chapter 9 - 2 Components and Phases • Components: The elements or compounds which are present in the mixture (e.g., Al and Cu) • Phases: The physically and chemically distinct material regions that result (e.g., a and b). AluminumCopper Alloy b (lighter phase) a (darker phase) Adapted from chapter-opening photograph, Chapter 9, Callister 3e. Chapter 9 - 3 Effect of T & Composition (Co) • Changing T can change # of phases: path A to B. • Changing Co can change # of phases: path B to D. B (100°C,70) D (100°C,90) 1 phase watersugar system Adapted from Fig. 9.1, Callister 7e. Temperature (°C) 100 2 phases L 80 (liquid) 60 L (liquid solution 40 i.e., syrup) + S (solid sugar) A (20°C,70) 20 2 phases 0 0 20 40 60 70 80 100 Co =Composition (wt% sugar) Chapter 9 - 4 Phase Equilibria Simple solution system (e.g., Ni-Cu solution) Crystal Structure electroneg r (nm) Ni FCC 1.9 0.1246 Cu FCC 1.8 0.1278 • Both have the same crystal structure (FCC) and have similar electronegativities and atomic radii (W. Hume – Rothery rules) suggesting high mutual solubility. • Ni and Cu are totally miscible in all proportions. Chapter 9 - 5 Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course: -binary systems: just 2 components. -independent variables: T and Co (P = 1 atm is almost always used). T(°C) • Phase Diagram for Cu-Ni system • 2 phases: 1600 1500 L (liquid) a (FCC solid solution) L (liquid) 1400 1300 a (FCC solid solution) 1200 1100 1000 0 20 40 60 80 • 3 phase fields: L L+a a Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys, P. Nash (Ed.), ASM International, Materials Park, OH (1991). 100 wt% Ni Chapter 9 - 6 Phase Diagrams: # and types of phases • Rule 1: If we know T and Co, then we know: --the # and types of phases present. A(1100°C, 60): 1 phase: a B(1250°C, 35): 2 phases: L + a 1600 L (liquid) B (1250°C,35) • Examples: T(°C) 1500 1400 1300 1200 Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys, P. Nash (Ed.), ASM International, Materials Park, OH, 1991). 1100 1000 Cu-Ni phase diagram a (FCC solid solution) A(1100°C,60) 0 20 40 60 80 100 wt% Ni Chapter 9 - 7 Phase Diagrams: composition of phases • Rule 2: If we know T and Co, then we know: --the composition of each phase. • Examples: T(°C) Cu-Ni system A TA Co = 35 wt% Ni tie line 1300 L (liquid) At T A = 1320°C: Only Liquid (L) B TB CL = Co ( = 35 wt% Ni) a At T D = 1190°C: (solid) 1200 D Only Solid ( a) TD Ca = Co ( = 35 wt% Ni) 20 3032 35 4043 50 At T B = 1250°C: CLCo Ca wt% Ni Both a and L Adapted from Fig. 9.3(b), Callister 7e. 9.3(b) is adapted from Phase Diagrams CL = C liquidus ( = 32 wt% Ni here) (Fig. of Binary Nickel Alloys, P. Nash (Ed.), ASM Ca = C solidus ( = 43 wt% Ni here) International, Materials Park, OH, 1991.) Chapter 9 - 8 Phase Diagrams: weight fractions of phases • Rule 3: If we know T and Co, then we know: --the amount of each phase (given in wt%). • Examples: Co = 35 wt% Ni At T A : Only Liquid (L) W L = 100 wt%, W a = 0 At T D: Only Solid ( a) W L = 0, Wa = 100 wt% At T B : Both a and L WL Wa S 43 35 73 wt % R + S 43 32 R = 27 wt% R +S Cu-Ni system T(°C) A TA tie line L (liquid) 1300 B R S TB 1200 D TD 20 3032 35 CLCo a (solid) 40 43 50 Ca wt% Ni Adapted from Fig. 9.3(b), Callister 7e. (Fig. 9.3(b) is adapted from Phase Diagrams of Binary Nickel Alloys, P. Nash (Ed.), ASM International, Materials Park, OH, 1991.) Chapter 9 - 9 The Lever Rule • Tie line – connects the phases in equilibrium with each other - essentially an isotherm T(°C) How much of each phase? Think of it as a lever (teeter-totter) tie line 1300 L (liquid) B TB a (solid) 1200 R 20 S R 30C C 40 C a L o wt% Ni WL Ma ML 50 S M a S M L R Adapted from Fig. 9.3(b), Callister 7e. C C0 ML S a ML Ma R S Ca CL Wa C CL R 0 R S Ca CL Chapter 9 - 10 Ex: Cooling in a Cu-Ni Binary • Phase diagram: Cu-Ni system. • System is: --binary i.e., 2 components: Cu and Ni. T(°C) L (liquid) 130 0 L: 35 wt% Ni a: 46 wt% Ni • Consider Co = 35 wt%Ni. Cu-Ni system A 35 32 --isomorphous i.e., complete solubility of one component in another; a phase field extends from 0 to 100 wt% Ni. L: 35wt%Ni B C 46 43 D 24 L: 32 wt% Ni 36 120 0 a: 43 wt% Ni E L: 24 wt% Ni a: 36 wt% Ni a (solid) 110 0 20 30 Adapted from Fig. 9.4, Callister 7e. 35 Co 40 50 wt% Ni Chapter 9 - 11 Cored vs Equilibrium Phases • Ca changes as we solidify. • Cu-Ni case: First a to solidify has Ca = 46 wt% Ni. Last a to solidify has Ca = 35 wt% Ni. • Fast rate of cooling: Cored structure • Slow rate of cooling: Equilibrium structure First a to solidify: 46 wt% Ni Last a to solidify: < 35 wt% Ni Uniform C a: 35 wt% Ni Chapter 9 - 12 Mechanical Properties: Cu-Ni System • Effect of solid solution strengthening on: --Ductility (%EL,%AR) 400 TS for pure Ni 300 TS for pure Cu 200 0 20 40 Cu 60 80 100 Ni Composition, wt% Ni Adapted from Fig. 9.6(a), Callister 7e. --Peak as a function of Co Elongation (%EL) Tensile Strength (MPa) --Tensile strength (TS) 60 %EL for pure Cu %EL for pure Ni 50 40 30 20 0 20 Cu 40 60 80 100 Ni Composition, wt% Ni Adapted from Fig. 9.6(b), Callister 7e. --Min. as a function of Co Chapter 9 - 13 Binary-Eutectic Systems has a special composition with a min. melting T. 2 components Cu-Ag system T(°C) Ex.: Cu-Ag system 1200 • 3 single phase regions L (liquid) 1000 (L, a, b) a L + a 779°C • Limited solubility: L+b b 800 T a: mostly Cu 8.0 71.9 91.2 E b: mostly Ag 600 • TE : No liquid below TE ab 400 • CE : Min. melting TE composition 200 • Eutectic transition L(CE) 0 a(CaE) + b(CbE) 20 40 60 CE 80 100 Co , wt% Ag Adapted from Fig. 9.7, Callister 7e. Chapter 9 - 14 EX: Pb-Sn Eutectic System (1) • For a 40 wt% Sn-60 wt% Pb alloy at 150°C, find... --the phases present: a + b T(°C) --compositions of phases: CO = 40 wt% Sn Ca = 11 wt% Sn Cb = 99 wt% Sn --the relative amount of each phase: Wa = C - CO S = b R+S Cb - Ca Pb-Sn system 300 200 L (liquid) a L+ a 18.3 150 100 99 - 40 59 = = 67 wt% 99 - 11 88 C - Ca Wb = R = O Cb - Ca R+S L+b b 183°C 61.9 R 97.8 S a+b = = 40 - 11 29 = = 33 wt% 99 - 11 88 0 11 20 Ca 40 Co 60 80 C, wt% Sn 99100 Cb Adapted from Fig. 9.8, Callister 7e. Chapter 9 - 15 EX: Pb-Sn Eutectic System (2) • For a 40 wt% Sn-60 wt% Pb alloy at 200°C, find... --the phases present: a + L T(°C) --compositions of phases: CO = 40 wt% Sn Ca = 17 wt% Sn CL = 46 wt% Sn --the relative amount of each phase: CL - CO 46 - 40 = Wa = CL - Ca 46 - 17 6 = = 21 wt% 29 Pb-Sn system 300 a 220 200 L (liquid) L+a R L+b b S 183°C 100 CO - Ca 23 = WL = = 79 wt% CL - Ca 29 a+b 0 17 20 Ca 40 46 60 Co CL 80 C, wt% Sn Adapted from Fig. 9.8, Callister 7e. Chapter 9 - 16 100 Microstructures in Eutectic Systems: I • Co < 2 wt% Sn • Result: --at extreme ends --polycrystal of a grains i.e., only one solid phase. T(°C) L: Co wt% Sn 400 L a L 300 a 200 (Pb-Sn System) a: Co wt% Sn TE a+ b 100 Adapted from Fig. 9.11, Callister 7e. L+ a 0 Co 10 20 30 Co, wt% Sn 2 (room T solubility limit) Chapter 9 - 17 Microstructures in Eutectic Systems: II L: Co wt% Sn • 2 wt% Sn < Co < 18.3 wt% Sn 400T(°C) • Result: Initially liquid + a then a alone finally two phases a polycrystal fine b-phase inclusions L 300 L+a a 200 TE a: Co wt% Sn a b 100 a+ b 0 Adapted from Fig. 9.12, Callister 7e. (sol. L a 10 20 Pb-Sn system 30 Co Co , wt% 2 limit at T room ) 18.3 (sol. limit at TE) Sn Chapter 9 - 18 Microstructures in Eutectic Systems: III • Co = CE • Result: Eutectic microstructure (lamellar structure) --alternating layers (lamellae) of a and b crystals. T(°C) L: Co wt% Sn 300 Pb-Sn system a 200 L+ a L Lb b 183°C TE 100 ab 0 20 18.3 Adapted from Fig. 9.13, Callister 7e. Micrograph of Pb-Sn eutectic microstructure 40 b: 97.8 wt% Sn a: 18.3 wt%Sn 60 CE 61.9 80 160 m Adapted from Fig. 9.14, Callister 7e. 100 97.8 C, wt% Sn Chapter 9 - 19 Lamellar Eutectic Structure Adapted from Figs. 9.14 & 9.15, Callister 7e. Chapter 9 - 20 Microstructures in Eutectic Systems: IV • 18.3 wt% Sn < Co < 61.9 wt% Sn • Result: a crystals and a eutectic microstructure L: Co wt% Sn T(°C) 300 L a L Pb-Sn system a 200 a L L+a R TE L+b b S S R primary a eutectic a eutectic b 0 20 18.3 Adapted from Fig. 9.16, Callister 7e. 40 60 61.9 Ca = 18.3 wt% Sn CL = 61.9 wt% Sn Wa = S = 50 wt% R+S WL = (1- Wa) = 50 wt% • Just below TE : a+b 100 • Just above TE : 80 Co, wt% Sn 100 97.8 Ca = 18.3 wt% Sn Cb = 97.8 wt% Sn Wa = S = 73 wt% R+S Wb = 27 wt% Chapter 9 - 21 Hypoeutectic & Hypereutectic 300 L T(°C) Adapted from Fig. 9.8, Callister 7e. (Fig. 9.8 adapted from Binary Phase Diagrams, 2nd ed., Vol. 3, T.B. Massalski (Editor-inChief), ASM International, Materials Park, OH, 1990.) a 200 L+ a a+b 20 40 hypoeutectic: Co = 50 wt% Sn a a (Pb-Sn System) 100 0 (Figs. 9.14 and 9.17 from Metals Handbook, 9th ed., Vol. 9, Metallography and Microstructures, American Society for Metals, Materials Park, OH, 1985.) L+b b TE a 60 80 eutectic 61.9 hypereutectic: (illustration only) b b a Adapted from Fig. 9.17, Callister 7e. Co, wt% Sn eutectic: Co = 61.9 wt% Sn a a 175 m 100 b b b b 160 m eutectic micro-constituent Adapted from Fig. 9.14, Callister 7e. Adapted from Fig. 9.17, Callister 7e. (Illustration only) Chapter 9 - 22 Intermetallic Compounds Adapted from Fig. 9.20, Callister 7e. Mg2Pb Note: intermetallic compound forms a line - not an area because stoichiometry (i.e. composition) is exact. Chapter 9 - 23 Eutectoid & Peritectic • Eutectic - liquid in equilibrium with two solids L cool a + b heat • Eutectoid - solid phase in equation with two solid phases intermetallic compound S2 S1+S3 - cementite cool a + Fe3C (727ºC) heat • Peritectic - liquid + solid 1 solid 2 (Fig 9.21) S1 + L S2 cool + L heat (1493ºC) Chapter 9 - 24 Eutectoid & Peritectic Cu-Zn Phase diagram Eutectoid transition + Peritectic transition + L Adapted from Fig. 9.21, Callister 7e. Chapter 9 - 25 Iron-Carbon (Fe-C) Phase Diagram T(°C) 1600 1200 a + Fe3C +L (austenite) 1000 a 800 600 120 m Result: Pearlite = alternating layers of a and Fe3C phases (Adapted from Fig. 9.27, Callister 7e.) S +Fe3C 727°C = Teutectoid R S 1 0.76 L+Fe3C R B 400 0 (Fe) A 1148°C 2 3 a+Fe3C 4 5 6 Fe3C (cementite) L + Fe3C -Eutectoid (B): L 1400 C eutectoid • 2 important points -Eutectic (A): 6.7 4.30 Co, wt% C Fe3C (cementite-hard) a (ferrite-soft) Adapted from Fig. 9.24,Callister 7e. Chapter 9 - 26 Hypoeutectoid Steel T(°C) 1600 L a a a +L 1200 (austenite) 1000 800 + Fe3C r s 727°C aRS w a =s/(r +s) 600 w =(1- wa ) 400 0 (Fe) pearlite a + Fe3C 1 C0 w pearlite = w 2 3 4 5 6.7 100 m w a =S/(R+S) w Fe3 =(1-w a ) C 6 Adapted from Figs. 9.24 and 9.29,Callister 7e. (Fig. 9.24 adapted from Binary Alloy Phase Diagrams, 2nd ed., Vol. 1, T.B. Massalski (Ed.-inChief), ASM International, Materials Park, OH, 1990.) Co , wt% C 0.76 a L+Fe3C 1148°C (Fe-C System) Fe3C (cementite) 1400 pearlite Hypoeutectoid steel proeutectoid ferrite Adapted from Fig. 9.30,Callister 7e. Chapter 9 - 27 Hypereutectoid Steel T(°C) 1600 L Fe3C +L 1200 (austenite) 1000 r 800 w Fe3C =r/(r +s) w =(1-w Fe3C ) a R 600 400 0 (Fe) pearlite L+Fe3C 1148°C +Fe3C 0.76 (Fe-C System) s S 1 Co w pearlite = w w a =S/(R+S) w Fe3C =(1-w a ) a +Fe3C 2 3 4 5 6 Fe3C (cementite) 1400 Adapted from Figs. 9.24 and 9.32,Callister 7e. (Fig. 9.24 adapted from Binary Alloy Phase Diagrams, 2nd ed., Vol. 1, T.B. Massalski (Ed.-inChief), ASM International, Materials Park, OH, 1990.) 6.7 Co , wt%C 60 mHypereutectoid steel pearlite proeutectoid Fe3C Adapted from Fig. 9.33,Callister 7e. Chapter 9 - 28 Example: Phase Equilibria For a 99.6 wt% Fe-0.40 wt% C at a temperature just below the eutectoid, determine the following a) composition of Fe3C and ferrite (a) b) the amount of carbide (cementite) in grams that forms per 100 g of steel c) the amount of pearlite and proeutectoid ferrite (a) Chapter 9 - 29 Chapter 9 – Phase Equilibria Solution: a) composition of Fe3C and ferrite (a) b) the amount of carbide (cementite) in grams that forms per 100 g of steel CO = 0.40 wt% C Ca = 0.022 wt% C CFe C = 6.70 wt% C 3 1600 1200 0.4 0.022 x 100 5.7g 6.7 0.022 L a 94.3 g L+Fe3C 1148°C (austenite) 1000 + Fe3C 800 Fe 3C 5.7 g +L Fe C (cementite) Fe 3C Co Ca 1400 x100 T(°C) Fe 3C a CFe3C Ca 727°C R S a + Fe3C 600 400 0 Ca CO 1 2 3 4 5 6 Co , wt% C 6.7 CFe 3C Chapter 9 - 30 Chapter 9 – Phase Equilibria c. the amount of pearlite and proeutectoid ferrite (a) note: amount of pearlite = amount of just above TE 1600 L 1400 T(°C) +L Co Ca x 100 51.2 g 1200 (austenite) a C Ca L+Fe3C 1148°C 1000 + Fe3C 800 727°C RS pearlite = 51.2 g proeutectoid a = 48.8 g a + Fe3C 600 400 0 1 Ca CO C 2 3 4 5 6 Co , wt% C Chapter 9 - 31 Fe C (cementite) Co = 0.40 wt% C Ca = 0.022 wt% C Cpearlite = C = 0.76 wt% C 6.7 Alloying Steel with More Elements Ti Mo Si W Cr Mn Ni wt. % of alloying elements Adapted from Fig. 9.34,Callister 7e. (Fig. 9.34 from Edgar C. Bain, Functions of the Alloying Elements in Steel, American Society for Metals, 1939, p. 127.) • Ceutectoid changes: Ceutectoid (wt%C) T Eutectoid (°C) • Teutectoid changes: Ni Cr Si Ti Mo W Mn wt. % of alloying elements Adapted from Fig. 9.35,Callister 7e. (Fig. 9.35 from Edgar C. Bain, Functions of the Alloying Elements in Steel, American Society for Metals, 1939, p. 127.) Chapter 9 - 32 Summary • Phase diagrams are useful tools to determine: --the number and types of phases, --the wt% of each phase, --and the composition of each phase for a given T and composition of the system. • Alloying to produce a solid solution usually --increases the tensile strength (TS) --decreases the ductility. • Binary eutectics and binary eutectoids allow for a range of microstructures. Chapter 9 - 33 ANNOUNCEMENTS Reading: Core Problems: Self-help Problems: Chapter 9 - 34