Chapter 2 - Materialteknologi
advertisement

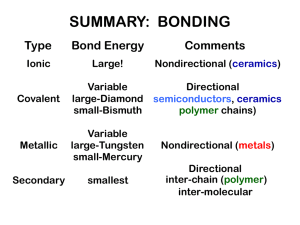
CHAPTER 2: Atomic Structure and Interatomic Bonding ISSUES TO ADDRESS... • What promotes bonding? • What types of bonds are there? • What properties are inferred from bonding? Chapter 2- 1 BOHR ATOM orbital electrons: n = principal quantum number 1 2 n=3 Adapted from Fig. 2.1, Callister 6e. Nucleus: Z = # protons = 1 for hydrogen to 94 for plutonium N = # neutrons Atomic mass A ≈ Z + N Chapter 2- 2 ELECTRON ENERGY STATES Electrons... • have discrete energy states • tend to occupy lowest available energy state. Adapted from Fig. 2.5, Callister 6e. Chapter 2- 3 STABLE ELECTRON CONFIGURATIONS Stable electron configurations... • have complete s and p subshells • tend to be unreactive. Adapted from Table 2.2, Callister 6e. Chapter 2- 4 SURVEY OF ELEMENTS • Most elements: Electron configuration not stable. Electron configuration 1s1 1s2 (stable) 1s22s1 1s22s2 Adapted from Table 2.2, 1s22s22p 1 Callister 6e. 1s22s22p 2 ... 1s22s22p 6 (stable) 1s22s22p 63s1 1s22s22p 63s2 1s22s22p 63s23p 1 ... 1s22s22p 63s23p 6 (stable) ... 1s22s22p 63s23p 63d 10 4s246 (stable) • Why? Valence (outer) shell usually not filled completely. Chapter 2- 5 THE PERIODIC TABLE • Columns: Similar Valence Structure Adapted from Fig. 2.6, Callister 6e. Electropositive elements: Readily give up electrons to become + ions. Electronegative elements: Readily acquire electrons to become - ions. Chapter 2- 6 ELECTRONEGATIVITY • Ranges from 0.7 to 4.0, • Large values: tendency to acquire electrons. Smaller electronegativity Larger electronegativity Adapted from Fig. 2.7, Callister 6e. (Fig. 2.7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3rd edition, Copyright 1939 and 1940, 3rd edition. Copyright 1960 by Cornell University. Chapter 2- 7 IONIC BONDING • • • • Occurs between + and - ions. Requires electron transfer. Large difference in electronegativity required. Example: NaCl Chapter 2- 8 EXAMPLES: IONIC BONDING • Predominant bonding in Ceramics NaCl MgO CaF2 CsCl H 2.1 Li 1.0 Be 1.5 Na 0.9 Mg 1.2 K 0.8 Ca 1.0 Sr 1.0 Rb 0.8 Cs 0.7 Fr 0.7 Ti 1.5 Cr 1.6 Ba 0.9 Fe 1.8 Ni 1.8 He O 3.5 Zn 1.8 As 2.0 F 4.0 Cl 3.0 Ne - Br 2.8 I 2.5 Kr Xe Rn - At 2.2 Ar - Ra 0.9 Give up electrons Acquire electrons Adapted from Fig. 2.7, Callister 6e. (Fig. 2.7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3rd edition, Copyright 1939 and 1940, 3rd edition. Copyright 1960 by Cornell University. Chapter 2- 9 COVALENT BONDING • Requires shared electrons • Example: CH4 C: has 4 valence e, needs 4 more H: has 1 valence e, needs 1 more Electronegativities are comparable. Adapted from Fig. 2.10, Callister 6e. Chapter 2- 10 EXAMPLES: COVALENT BONDING H2 H 2.1 Li 1.0 Na 0.9 K 0.8 Rb 0.8 Cs 0.7 Sr 1.0 Ba 0.9 Fr 0.7 Ra 0.9 • • • • C(diamond) SiC Be 1.5 Mg 1.2 Ca 1.0 column IVA H2O Ti 1.5 Cr 1.6 Fe 1.8 F2 He O 2.0 C 2.5 Ni 1.8 Zn 1.8 Ga 1.6 Si 1.8 Ge 1.8 As 2.0 Sn 1.8 Pb 1.8 F 4.0 Cl 3.0 Ne - Br 2.8 Ar Kr - I 2.5 Xe - At 2.2 Rn - Cl2 GaAs Adapted from Fig. 2.7, Callister 6e. (Fig. 2.7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3rd edition, Copyright 1939 and 1940, 3rd edition. Copyright 1960 by Cornell University. Molecules with nonmetals Molecules with metals and nonmetals Elemental solids (RHS of Periodic Table) Compound solids (about column IVA) Chapter 2- 11 METALLIC BONDING • Arises from a sea of donated valence electrons (1, 2, or 3 from each atom). Adapted from Fig. 2.11, Callister 6e. • Primary bond for metals and their alloys Chapter 2- 12 SECONDARY BONDING Arises from interaction between dipoles • Fluctuating dipoles Adapted from Fig. 2.13, Callister 6e. • Permanent dipoles-molecule induced -general case: -ex: liquid HCl Adapted from Fig. 2.14, Callister 6e. Adapted from Fig. 2.14, Callister 6e. -ex: polymer Chapter 2- 13 SUMMARY: BONDING Type Bond Energy Comments Ionic Large! Nondirectional (ceramics) Covalent Variable Directional large-Diamond semiconductors, ceramics small-Bismuth polymer chains) Metallic Variable large-Tungsten small-Mercury Nondirectional (metals) smallest Directional inter-chain (polymer) inter-molecular Secondary Chapter 2- 14 PROPERTIES FROM BONDING: TM • Bond length, r F • Melting Temperature, Tm F r • Bond energy, Eo Tm is larger if Eo is larger. Chapter 2- 15 PROPERTIES FROM BONDING: E • Elastic modulus, E Elastic modulus F L =E Ao Lo • E ~ curvature at ro Energy unstretched length ro r E is larger if Eo is larger. smaller Elastic Modulus larger Elastic Modulus Chapter 2- 16 PROPERTIES FROM BONDING: a • Coefficient of thermal expansion, a coeff. thermal expansion L = a(T2-T1) Lo • a ~ symmetry at ro a is larger if Eo is smaller. Chapter 2- 17 SUMMARY: PRIMARY BONDS Ceramics (Ionic & covalent bonding): Metals (Metallic bonding): Polymers (Covalent & Secondary): Large bond energy large Tm large E small a Variable bond energy moderate Tm moderate E moderate a Directional Properties Secondary bonding dominates small T small E large a Chapter 2- 18 ANNOUNCEMENTS Reading: Core Problems: Self-help Problems: Chapter 2- 0