Chapter 9: Phase Diagrams
advertisement
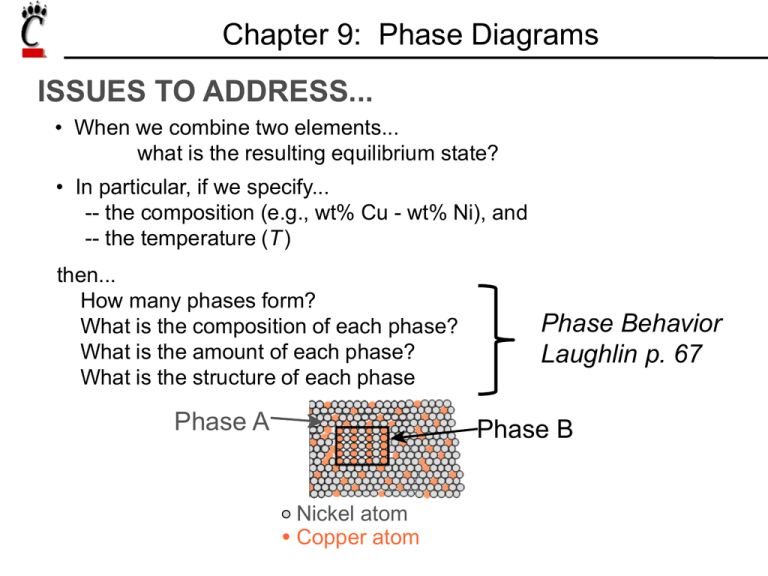
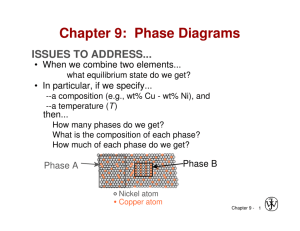
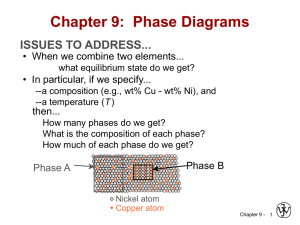
Chapter 9: Phase Diagrams ISSUES TO ADDRESS... • When we combine two elements... what is the resulting equilibrium state? • In particular, if we specify... -- the composition (e.g., wt% Cu - wt% Ni), and -- the temperature (T) then... How many phases form? What is the composition of each phase? What is the amount of each phase? What is the structure of each phase Phase A Phase Behavior Laughlin p. 67 Phase B Nickel atom Copper atom Components and Phases • Components: The elements or compounds which are present in the alloy (e.g., Al and Cu) • Phases: The physically and chemically distinct material regions that form (e.g., α and β). Optical Mettalography α (lighter phase) β (darker phase) 1µm Aluminum-Copper Alloy Solubility Limit • Solution – solid, liquid, or gas solutions, single phase • Mixture – more than one phase Sugar/Water Phase Diagram • Solubility Limit: Question: What is the solubility limit for sugar in water at 20ºC? Temperature (ºC) Maximum concentration for which only a single phase solution exists. 100 Solubility Limit 80 L (liquid) 60 40 L (syrup) 20 0 20 + S (solid sugar) 40 6065 80 100 Composition (wt% sugar) Problem 9.2: At 170°C, what is the maximum solubility (a) of Pb in Sn? (b) of Sn in Pb? The lead-tin phase diagram is shown in the Animated Figure 9.8. Isomorphous Binary Phase Diagram Cu-Ni phase diagram 1600 • System is: 1500 -- binary -- isomorphous i.e., complete solubility of one component in another 1400 T(ºC) 2 components: Cu and Ni. L (liquid) 1300 α 1200 1100 1000 solid solution 0 20 40 60 wt% Ni 80 100 Criteria for Solid Solubility: Hume–Rothery rules •Same crystal structure •Similar electronegativities •Similar atomic radii Crystal Structure electroneg r (nm) Ni FCC 1.9 0.1246 Cu FCC 1.8 0.1278 • Ni and Cu are totally soluble in one another for all proportions. Structure BCC FCC Periodic Table Determination of phases present • If we know T and Co, then we know which phases are present. Cu-Ni phase diagram • Examples: B(1250ºC, 35 wt% Ni): 2 phases: L + α L 1400 T(ºC) A(1100ºC, 60 wt% Ni): 1 phase: α 1500 1300 B (1250ºC,35) 1600 α 1200 1100 1000 A(1100ºC,60) 0 20 40 60 wt% Ni 80 100 Determination of phase compositions: Lever Rule Tie line –– also sometimes called an isotherm T(ºC) What fraction of each phase? Think of the tie line as a lever (teeter-totter) tie line 1300 L B TB Mα ML α 1200 R 20 30CL S C0 40 Cα 50 wt% Ni C C0 ML S WL ML M R S C CL R S M x S ML x R C0 CL R W R S C CL Slow Cooling of a Cu-Ni Alloy C0 = 35 wt% Ni alloy T(ºC) L L: 35wt%Ni 130 0 L: 35 wt% Ni α: 46 wt% Ni 35 46 32 24 43 L: 32 wt% Ni 36 : 43 wt% Ni 120 0 L: 24 wt% Ni : 36 wt% Ni α 110 0 20 30 35 C0 40 50 wt% Ni Cored vs Equilibrium Structures Uniform C: 35 wt% Ni T(ºC) L 130 0 35 46 32 24 43 36 120 0 First α to solidify: 46 wt% Ni α Last to solidify: < 35 wt% Ni 110 0 20 30 35 C0 40 50 wt% Ni Mechanical Properties: Cu-Ni System • Effect of solid solution strengthening on: -- Ductility (%EL) 400 TS for pure Ni 300 TS for pure Cu 200 0 20 40 Cu 60 80 100 Ni Composition, wt% Ni Elongation (%EL) Tensile Strength (MPa) -- Tensile strength (TS) 60 %EL for pure Cu %EL for pure Ni 50 40 30 20 0 20 Cu 40 60 80 100 Ni Composition, wt% Ni Binary-Eutectic Systems =low melting 2 components Cu-Ag system Ex.: Cu-Ag system • 3 single phase regions (L, α, β) 1200 L (liquid) 1000 TE 800 α L+α L+β β 779ºC 8.0 71.9 91.2 600 α +β 400 200 0 20 40 60 CE 80 wt% Ag Eutectic Phase Reaction: L(71.9 wt% Ag) cooling heating (8.0 wt% Ag) (91.2 wt% Ag) 100 Microstructural Development in Eutectic Systems I Pb-Sn • For alloys for which C0 < 2 wt% Sn • Result: at room temperature -- polycrystalline with grains of a phase having composition C0 T(ºC) L: C0 wt% Sn 400 L α L 300 200 TE 100 α L+ α α: C0 wt% Sn α+β 0 10 20 30 C0 wt% Sn 2 (room T solubility limit) Microstructural Development in Eutectic Systems II • For alloys for which 400 2 wt% Sn < C0 < 18.3 wt% Sn • Result: at temperatures in α + β range 300 -- polycrystalline with a grains and small β-phase particles 200 L: C0 wt% Sn T(ºC) L L α L +α a: C0 wt% Sn α TE α β 100 α+ β 0 10 20 30 C0 C, wt% 2 (sol. limit at T room ) 18.3 (sol. limit at TE) Sn Microstructural Development in Eutectic Systems III • For alloy of composition C0 = CE • Result: Eutectic microstructure (lamellar structure) -- alternating layers (lamellae) of α and β phases. T(ºC) Micrograph of Pb-Sn eutectic microstructure L: C0 wt% Sn 300 α 200 L+ α L β 183ºC TE 100 β: 97.8 wt% Sn α: 18.3 wt%Sn 0 20 18.3 40 60 CE 61.9 80 100 97.8 wt% Sn 160 μm Lamellar Eutectic Structure Microstructural Development in Eutectic Systems IV • For alloys for which 18.3 wt% Sn < C0 < 61.9 wt% Sn • Result: α phase particles and a eutectic microconstituent • Just above TE : L: C0 wt% Sn L T(ºC) 300 L Cα = 18.3 wt% Sn CL = 61.9 wt% Sn S = 0.50 = Wα R+S WL = (1- Wα ) = 0.50 α L+ α α 200 R TE S S R • Just below TE : 100 primary α eutectic α eutectic β 0 20 18.3 40 60 61.9 80 wt% Sn 100 97.8 Cα = 18.3 wt% Sn Cβ = 97.8 wt% Sn Wα = S = 0.73 R+S Wβ = 0.27 Hypoeutectic & Hypereutectic 300 L T(ºC) α 200 L+ α L+β β TE System) α+β 100 0 20 40 hypoeutectic: C0 = 50 wt% Sn α α α 60 80 eutectic 61.9 C, wt% Sn hypereutectic: (illustration only) eutectic: C0 = 61.9 wt% Sn β β α α β α 175 mm 100 160 mm eutectic micro-constituent β β β Intermetallic Compounds Mg2Pb Note: intermetallic compound exists as a line - not an area – because stoichiometry (i.e. composition of a compound) is fixed. Eutectic, Eutectoid, & Peritectic • Eutectic - liquid transforms to two solid phases L cool S1+S3 (For Pb-Sn, 183ºC, 61.9 wt% Sn) heat • Eutectoid –all solid phases intermetallic compound - cementite S2 S1+S3 cool ϒ heat α+ Fe3C (For Fe-C, 727ºC, 0.76 wt% C) • Peritectic - liquid and one solid phase transform to a second solid phase S1 + L S2 δ +L cool heat ϒ (For Fe-C, 1493ºC, 0.16 wt% C) • Peritectoid – all solid phases S1 + S2 S3 Eutectoid & Peritectic Cu-Zn Phase diagram Peritectic transformation γ + L Eutectoid transformation δ γ+ε δ Iron-Carbon (Fe-C) Phase Diagram T(ºC) 1600 δ L → γ + Fe3C - Eutectoid (B): γ → α + Fe3C L 1400 1200 γ +L γ (austenite) γ γ γ γ 1000 800 α 120 mm Result: Pearlite = alternating layers of α and Fe3C phases L+Fe3C γ +Fe3C 727ºC = T eutectoid B 600 400 0 (Fe) A 1148ºC α +Fe3C 1 0.76 2 3 4 4.30 5 6 wt% C Fe3C (cementite-hard) α (ferrite-soft) Fe3C (cementite) - Eutectic (A): 6.7 Hypereutectoid Steel T(ºC) 1600 δ γγ γ γ γ γ Fe3C 1200 proeutectoid Fe3C L 1400 γ +L γ (austenite) L+Fe3C 1148ºC 1000 γ +Fe3C 800 60 μm a 600 400 0 (Fe) pearlite pearlite α +Fe3C 0.76 γ 1 C0 2 3 4 5 6 6.7 C, wt%C T(ºC) 1600 δ L 1400 γ γ γ γ 1200 γ +L γ (austenite) γ γ γ γ α γ + Fe3C 800 pearlite proeutectoid ferrite 727ºC α 600 400 0 (Fe) C0 pearlite 100 μm L+Fe3C 1148ºC 1000 α + Fe3C 1 0.76 α Hypoeutectoid Steel 2 3 4 5 6 6.7 C, wt% C For a 99.6 wt% Fe-0.40 wt% C steel at a temperature just below the eutectoid, determine the following: a) The compositions of Fe3C and ferrite (α). b) The amount of cementite (in grams) that forms in 100 g of steel. c) The amounts of pearlite and proeutectoid ferrite (α) in the 100 g. Solution to Example Problem a) The compositions of Fe3C and ferrite (α). RS tie line just below the eutectoid Cα = 0.022 wt% C CFe3C = 6.70 wt% C pearlite b) Wight Fraction of cementite 1600 δ R C C 0 R S CFe3C C 0.40 0.022 0.057 6.70 0.022 T(ºC) 1200 γ γ +L 1000 Amount of Fe3C in 100 g = (100 g)WFe3C = (100 g)(0.057) = 5.7 g L+Fe3C 1148ºC (austenite) Fe C (cementite) WFe3C L 1400 γ + Fe3C 800 727ºC R S α + Fe3C 600 400 0 Cα C0 1 2 3 4 C, wt% C 5 6 6.7 CFe 3C The amounts of pearlite in the 100 g. c) Using the VX tie line just above the eutectoid and realizing that C0 = 0.40 wt% C Cα = 0.022 wt% C Cpearlite = Cγ = 0.76 wt% C α pearlite 1600 V C C 0 V X C C 0.40 0.022 0.512 0.76 0.022 Amount of pearlite in 100 g = (100 g)Wpearlite T(ºC) 1200 γ γ +L L+Fe3C 1148ºC (austenite) 1000 γ + Fe3C 800 727ºC VX + Fe3C 600 400 0 1 Cα C0 Cγ = (100 g)(0.512) = 51.2 g 2 3 4 C, wt% C 5 6 Fe C (cementite) Wpearlite L 1400 6.7 Alloying with Other Elements Ti Mo Si W Cr Mn Ni wt. % of alloying elements • Ceutectoid changes: Ceutectoid (wt% C) T Eutectoid (ºC) • Teutectoid changes: Ni Cr Si Ti Mo W Mn wt. % of alloying elements VMSE: Interactive Phase Diagrams Microstructure, phase compositions, and phase fractions respond interactively Change alloy composition Summary • Phase diagrams are useful tools to determine: -- the number and types of phases present, -- the composition of each phase, -- and the weight fraction of each phase given the temperature and composition of the system. • The microstructure of an alloy depends on -- its composition, and -- whether or not cooling rate allows for maintenance of equilibrium. • Important phase diagram phase transformations include eutectic, eutectoid, and peritectic. Effect of Temperature & Composition • Altering T can change # of phases: path A to B. • Altering C can change # of phases: path B to D. B (100ºC,C = 70) D (100ºC,C = 90) 1 phase Temperature (ºC) 100 2 phases L 80 (liquid) 60 L (liquid solution 40 i.e., syrup) + S (solid sugar) A (20ºC,C = 70) 20 2 phases 0 0 20 40 60 70 80 100 C = Composition (wt% sugar) EX 1: Pb-Sn Eutectic System • For a 40 wt% Sn-60 wt% Pb alloy at 150ºC Cα = 11 wt% Sn Pb-Snsystem T(ºC) Cβ = 99 wt% Sn 300 -- the relative amount of each phase 200 = 40 - 11 29 = = 0.33 99 - 11 88 α L+ α 100 L+β β 183ºC 18.3 150 Cβ - C0 S = W = R+S Cβ - Cα α 99 - 40 59 = = = 0.67 99 - 11 88 C0 - Cα R Wβ = = Cβ - Cα R+S L (liquid) 61.9 R 97.8 S α+β 0 11 20 Cα 40 C0 60 80 wt% Sn 99100 Cβ EX 2: Pb-Sn Eutectic System • For a 40 wt% Sn-60 wt% Pb alloy at 220ºC Ca = 17 wt% Sn CL = 46 wt% Sn the relative amount of each phase CL - C0 46 - 40 = Wa = CL - Cα 46 - 17 6 = = 0.21 29 C0 - Cα 23 = = 0.79 WL = CL - Cα 29 T(ºC) 300 α 220 200 L+ α R L L+β β S 183ºC 100 a +b 0 17 20 Cα 40 46 60 C0 CL 80 wt% Sn 100