Unit 1 Section B: Counting Subatomic Particles Worksheet 1. boron

Name: _________________________________________________
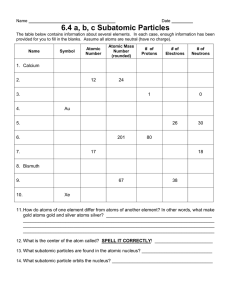
Unit 1 Section B: Counting Subatomic Particles Worksheet
Using a periodic table, fill in the chart with the correct information. Assume that all atoms are electrically neutral.
Name Symbol Atomic
Number
M ass
Number
# of
Protons
# of
Neutrons
# of
Electrons
1. boron
2. zinc
B 5 11 5 6
31
5
3.
4.
5.
6.
7.
8. fermium
9. cesium
10.
K
U
22
42
42
122
257
97
47
27
146
60
79
11.
12. krypton
13.
14. N
86
120
222
15
78
47
15. 7 7
1. What similarities exist between the answers to questions 14 and 15 on the table?
______________________________________________________________________________
55
51
______________________________________________________________________________
2. What differences exist between the answers to questions 14 and 15 on the table?
______________________________________________________________________________
______________________________________________________________________________
3. What term can be applied to atoms like those described in questions 14 and 15 on the table?
______________________________________________________________________________
______________________________________________________________________________
4. What is the difference between mass number (used in this exercise) and atomic weight (found on the periodic table)?
_____________________________________________________________________________________
_______________________________________________________________________