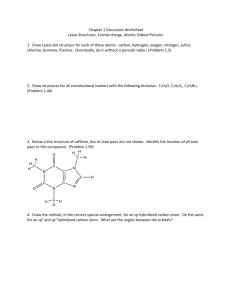
2 The other sp orbitals hold the oxygen lone pairs
advertisement

Worksheet 14 - Hybridization The hybridized molecular orbitals have different shapes and energy levels than the atomic orbitals. The number of molecular orbitals created by hybridization depends on the number of atomic orbitals that are mixed to form them. In forming sp3 hybridized orbitals, four atomic orbitals are mixed, one s and three p. The energy diagram for this process is shown below. The hybridized orbitals are higher in energy than the s orbital, but lower in energy than the p orbitals. p energy sp3 hybridization s atomic orbitals hybridized orbitals Carbon has 4 valence electrons. Add these electrons to the atomic and molecular orbitals. This hybridization gives tetrahedral geometry. With this hybridization, C will form four equivalent σ bonds. Draw a similar energy diagram for sp3 hybridized oxygen. p energy sp3 hybridization s How many σ bonds will be formed? How are the other sp3 orbitals used? 2 The other sp3 orbitals hold the oxygen lone pairs Do the same for sp3 hybridized nitrogen. p energy sp3 s hybridization In some Lewis structures, there are only three equivalent bonds formed. To create three equivalent hybridized orbitals, mix three atomic orbitals. Draw and name the orbitals formed in this hybridization, then add the electrons for sulfur. Since the hybridized orbitals are close in energy, every orbital is filled with one electron before electrons are paired. p p sp2 energy hybridization s The hybridized orbitals will form __1___ σ bond(s). The unhybridized orbital will form __1___ π bond(s). There will be __2___ lone pair(s). This hybridization gives trigonal planar geometry. In linear molecules, like CO2, the central atom has only two equivalent bonding orbitals. Draw the energy levels and name the orbitals formed in this hybridization. p p sp energy hybridization s Fill in the electrons for carbon and determine the number and type of bonds formed. There will be 2 sigma bonds (with sp) and 2 pi bonds (with p) In CO2, determine the hybridization of the oxygen atoms. Complete the energy diagram for the oxygens. Draw the structure of CO2. p p sp2 energy : : hybridization : : s O C O Each oxygen atom forms 1 sigma bond (with the sp2) and 1 pi bond (with the p) with the sp and p orbitals on the carbon atom In atoms with n=3 or larger, the d orbitals can also be hybridized. In molecules with five molecular orbitals, five atomic orbitals are mixed: _d_ + _s_ + _p_ + _p_ + _p_ This will give trigonal bipyramidal geometry and is called dsp3 hybridization. Finally, molecules with octahedral geometry, will have __6 __ molecular orbitals. This 2 3 hybridization is called __d sp ______ . Shown below is a portion of the chart from Worksheet 13. Fill in the hybridization for each of the compounds. compound bonds lone pairs geometry shape hybridization bonds + lone pairs = 6 SF6 6 0 octahedral octahedral d2sp3 bonds + lone pairs = 4 NH3 3 1 tetrahedral trigonal pyramidal sp3 bonds + lone pairs = 6 ICl4 - 4 2 octahedral square planar d2sp3 bonds + lone pairs = 4 CF4 4 0 tetrahedral tetrahedral sp3 bonds + lone pairs = 3 SO3 3 0 trigonal planar trigonal planar sp2 bonds + lone pairs = 5 SF4 4 1 trigonal bipyramidal seesaw dsp3 bonds + lone pairs = 2 CO2 2 0 linear linear sp bonds + lone pairs = 4 H2O 2 2 tetrahedral V-shaped sp3 bonds + lone pairs = 3 NO2 - 2 1 trigonal planar V-shaped sp2 Fill in the chart below and then complete the Lewis structures for the molecules shown below and fill in those charts. element Lewis symbol # bonds # lone pairs C 4 0 C N N H H O O Halogen F 1 H O 3 1 1 0 2 2 1 3 σ bonds __7__ H C C O H 3 2 H acetic acid π bonds __ 1__ atom # 1 2 3 bond angle hybridization 120 sp2 109 sp3 120 sp2 H H C C H 3 C 2 N 1 σ bonds _6___ π bonds _3___ acrylonitrile atom # 1 2 3 bond angle hybridization 180 sp 180 sp 120 sp2 H 2 O H C H H C N C C N C H H C C N 1 O N H H C 3 H H caffeine σ bonds 25__ π bonds 4__ atom # 1 2 3 bond angle hybridization 120 sp2 120 sp2 109 sp3 The molecule shown to the left is riboflavin (vitamin B2). Answer the following questions about its structure. a) how many carbons are sp3 hybridized? 8 H H O C H H C O H H O C H H C O H H H H C H sp hybridized? 0 H C H H H C H H H C sp2 hybridized? 10 C C C C C C N N C C H N C How many nitrogens are sp3 hybridized? 2 b) C O N sp2 hybridized? 2 sp hybridized? 0 H O How many oxygens are sp3 hybridized? 4 c) sp2 hybridized? 2 sp hybridized? 0 d) How many σ bonds are there in total? 52 e) How many π bonds are there in total? 7 f) Which of the three rings are planar? The ring on the left, in which all carbon atoms are sp2 hybridized The acetate ion, C2H3O2- , has both oxygens bonded to the same carbon. a) Draw the Lewis structure and all resonance forms. H O C C O H H O sp3 sp2 H C C O H sp3 H sp2 b) Label the hybridization around each carbon. c) Pick one resonance structure and label the hybridization of each oxygen. d) How many σ and π bonds are present? 6 sigma bonds, 1 pi bond e) Which atom carries the formal negative charge? the oxygen with 3 lone pairs