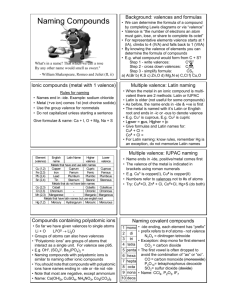
SCH 4U Naming Review 1. Naming Binary Compounds *Binary
advertisement

SCH 4U Naming Review 1. Naming Binary Compounds *Binary compounds are compounds that contain two elements. a) Binary Covalent Compounds: o Always two non-metals. o Use the prefix system. (1) The element with the lowest electronegativity is written first (the element further to the left in the periodic table - if the elements are in the same group, write the element which is lower in the group first) Remove the ending from the name of the second element, and replace is with “ide”. The number of atoms of each element is indicated prefixes: (2) (3) 1 mono- 2 di- Notes: Examples: CO N2S5 SO3 3 tri- 4 tetra- 5 penta- 6 hexa- 7 hepta- 8 octa- 9 nona- 10 deca- * “mono” is never used with the first element in the compound’s name * when a prefix ending in “o” or “a” is followed by “oxygen” or “oxide”, the “o” or “a” of the prefix is dropped ____________________ ____________________ ____________________ phosphorus trichloride nitrogen dioxide oxygen difluoride __________ __________ __________ b) Binary Ionic Compounds: o Always a metal and a non-metal. o Use the stock system or classical system for multivalent elements. o DO NOT use prefixes! Stock System (Roman Numerals) (1) (2) (3) Examples CuCl2 KBr FeCl3 The name of the positive ion is written first. The name of the negative ion follows with the ending replaced by the suffix “-ide”. Roman numerals are used to indicate the valence (charge) of the element if it has more than one valence (stock system) ____________________ ____________________ ___________________ tin(IV) oxide calcium iodide platinum(IV) fluoride __________ __________ __________ Classical Naming System (“ous-ic” system) • oldest system and not commonly used • derives form the Latin names of elements • used to name compounds where the first element may have more than one valence. (1) When the metal exhibits its lower valence, the ending –ous is added to the latin root of the name. (2) When the metal exhibits its higher valence, the ending –ic is used in the same way. Element Symbol iron copper tin lead gold Fe Cu Sn Pb Au Latin Name ferrum cuprum stannum plumbum aurum Example FeCl2 ___________________ Lower Valence 2 1 2 2 1 Ion Name ferrous cuprous stannous plumbous aurous FeCl3 Ion Name ferric cupric stannic plumbic auric Higher Valence 3 2 4 4 3 ___________________ 2. Naming Polyatomic Compounds • polyatomic ions: formed from more than one atom joined together by covalent bonds. phosphPO23Hypo____ite PO33____ite PO43____ate Per______ate PO53- nitrNONO2NO3NO4- Other polyatomic ions: sulf- carbon- iodSO22- CO2IOSO32- CO22- IO2SO42- CO32- IO3SO52- CO42- IO4- bromBrOBrO2BrO3BrO4- chlorClO2 less O ClO2 1 less O ClO3 know this ClO4- 1 more O see list of common ions The ionic rules for naming are used. Name the polyatomic ion as a unit. Name the following or give the formula: sodium chlorate tin(II) acetate zinc hydroxide ____________________ ____________________ ____________________ Example hypochlorite chlorite chlorate perchlorate 3. Naming Acids a) Binary Acids: compounds formed of hydrogen plus one other element. hydro ________ic acid Examples: HCl(aq) HF(aq) HI(aq) HBr(aq) hydrochloric acid ____________________ ____________________ ____________________ b) Oxyacids: acid formed by combining hydrogen with a polyatomic ion containing oxygen Example 2H+ + SO42- → H2SO4 Ion Formula SO22SO32SO42SO52- Name of Ion hyposulfite sulfite sulfate persulfate sulfuric acid Acid Formula H2SO2 H2SO3 H2SO4 H2SO5 Name the following or give the formula: HNO3 ____________________ HCl ____________________ HC2H3O2 ____________________ H2S ____________________ Name of Acid hyposulfurous acid sulfurous acid sulfuric acid persulfuric acid hydrofluoric acid phosphorous acid carbonic acid hydroiodic acid _________ _________ _________ _________ 4. Hydrated Salts: • salt crystals that contain molecules of water within the crystal structure Example: CuSO4•5H2O copper(II) sulfate pentahydrate 5. Acid Salts: • a compound formed when only some of the hydrogen atoms in an acid are replaced by a metal Example: H2CO3 → carbonic acid NaHCO3 sodium hydrogen carbonate