Naming Chemical Compounds Worksheet
advertisement

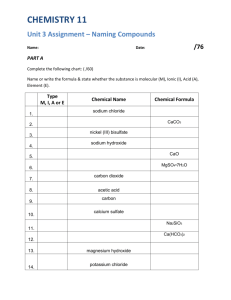
Assignment 1.3: Naming Compounds To do this assignment you will need to have your periodic table, and a table of polyatomic ions (use pages 488, 489 and 491 as your resource): Part 1: Forming and Naming Binary Compounds Use your periodic table to fill in the following table: Metal Nonmetal potassium lithium aluminum hydrogen magnesium sodium calcium sodium copper (II magnesium aluminum sulphur phosphorus chlorine fluorine sulphur oxygen chlorine nitrogen selenium phosphorus sulphur metal ion symbol K+ nonmetal ion symbol S2- Formula Name of Formula K2S potassium sulphide Part 2: Forming and Naming Polyatomic compounds Using the table for polyatomic ions and your periodic table to fill the following table: Cation (+ion) aluminum potassium sodium ammonium calcium magnesium Anion (- ion) hydrogen sulphide nitrate hydrogen carbonate chloride phosphate sulphate cation symbol anion symbol Formula Name of Formula Part 3: Naming and Determining Bonding Properties of Compounds 1. To review the concepts of molecular and ionic compounds please complete the table below: A. Name the following compounds and indicate whether they are ionic or molecular: (Hint: Some of these compounds have common names you will have to look up in your book and some are elemental compounds, so be careful. Use page 489 for help). Compound Name of Compound Ionic or Molecular? C6 H12O6 BaSe Cl2 SO3 Fe (NO3)2 Cs2O Li3N Al4C3 NaBr N2 O5 C3CH2OH B. Write the following formulas for the following compounds. Indicate whether they are ionic or molecular: Compound Sodium iodide chromium (III) carbonate barium phosphate silicon tetrahydride tin (IV) chloride lead (II) sulphite zinc hydroxide sulphur dioxide oxygen dibromide carbon tetrafluoride Chemical Formula Ionic or Molecular? Part 4: Making a Useful Key for Naming compounds A dichotomous key gives you two choices at each level based upon characteristics of the concept you are studying. In this case, you want to design a simple key to help you whenever you are naming chemical compounds. Please fill in the blanks with the information needed: