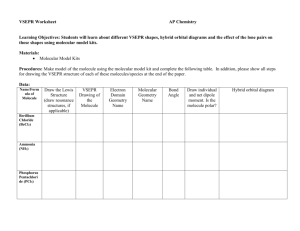
7.53 Using the molecular orbital energy ordering for second
advertisement

7.53 Using the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the π2p orbitals lie at lower energy than the σ2p, predict the bond order in a molecule or ion with each of the following numbers of total valence electrons.(Use the drawing MO energy diagrams) Will the molecule or ion be diamagnetic or paramagnetic? Determine the bond order in a molecule or ion with 4 valence electrons. Will this molecule or ion be diamagnetic or paramagnetic? 7.55 Use molecular orbital theory to predict whether or not each of the following molecules or ions should exist in a relatively stable form. H2−2 F2−2