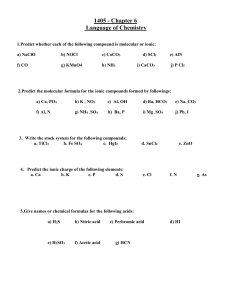
Ionic Compound Formula Writing Activity Guide
advertisement

IONIC SPEED DATING – Instructor Information This activity is used as a reinforcement activity following my use of JCE Classroom Activity #113: An Interlocking Building Block Activity in Writing Formulas of Ionic Compounds. Ruddick, Kristie R and Parrill, Abby L, An Interlocking Building Block Activity in Writing Formulas of Ionic Compounds, Journal of Chemical Education 2012, 89 (11), pp 1436-1438. Materials: Blue building blocks to represent cations & Red building blocks to represent anions. For one set each of A – D as described in the table below, you will need Blue: three 3 x 1, nine 2 x 1, nine 1 x 1 Red: six 3 x 1, eight 2 x 1, nine 1 x 1 The knobs on each block represent the valency or charge of the related ion. For example, a 1 x 3 blue block can represent an aluminum ion with a charge of 3+ and a 1 x 1 red block can represent a chloride ion with a charge of 1-. Therefore, you will need 3 red (chloride ion) blocks to neutralize the blue (aluminum ion) block. The neutral formula units are rectangular and are made up of the lowest whole number ratio of blocks. (All blue blocks must be in a single row and all red blocks must be in a single row.) I make up a variety of sets of blocks and keep them in baggies labeled Set A, Set B, Set C and Set D. Students will rotate through the sets with only 2 minutes per set. (Create as many sets as you like. Sets A – D are described below.) I created 6 baggies of each set and have students rotate through sets A – B. Remind students to write the formulas and names of the compounds as they manipulate the sets. They will not have time to complete that part later. (I do allow for a minute between sets to allow students to complete the formula and name of the last compound they built.) Students should disassemble the formula units before they move to the next set. They may use ions more than once in each set, but they must be able to build a complete formula unit in order to include it in the data table (ie. Ca2N3 cannot be made from set A because there is only one N 3- available). Use permanent marker to write the ion names on the appropriate red or blue blocks. Remind students that there must be a blue line of blocks and a red line of blocks for each formula unit. Set A Blue (cations) 3 Na+, 2 Ca2+ Red (anions) 2 P3-, 3 Cl-, 1 N3- B 2 Ca2+, 2 Al3+, 2 K+ 3 S2-, 2 Br- C 3 Ba2+, 1 Ca2+, 2 Li1+ 3 S2-, 2 F1-, 1 N3- D 2 Ag+, 1 Al3+, 1 Ba2+ 2 O2-, 2 I1-, 2 P3- Possible compounds Na3P, NaCl, CaCl2, Ca3P2, Na3N CaBr2, Al2S3, CaBr2, K2S, KBr, CaS BaS, BaF2, CaF2, CaS, Li2S, LiF Ag2O, AgI, AlP, BaO, BaI2 The number of compounds found per set may vary per group because of the time restraint. The teacher may prefer to ignore the time involved and require students to complete only one set or a combination of sets. Cullen 2013, ChemEdX.org: This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ or send a letter to Creative Commons, 444 Castro Street, Suite 900, Mountain View, California, 94041, USA.