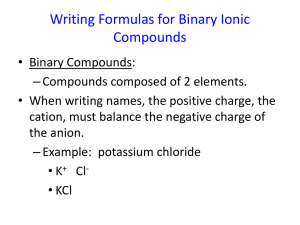
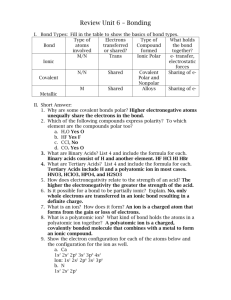
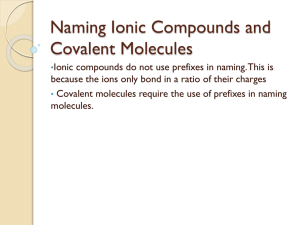
Integrated Biological Science
advertisement
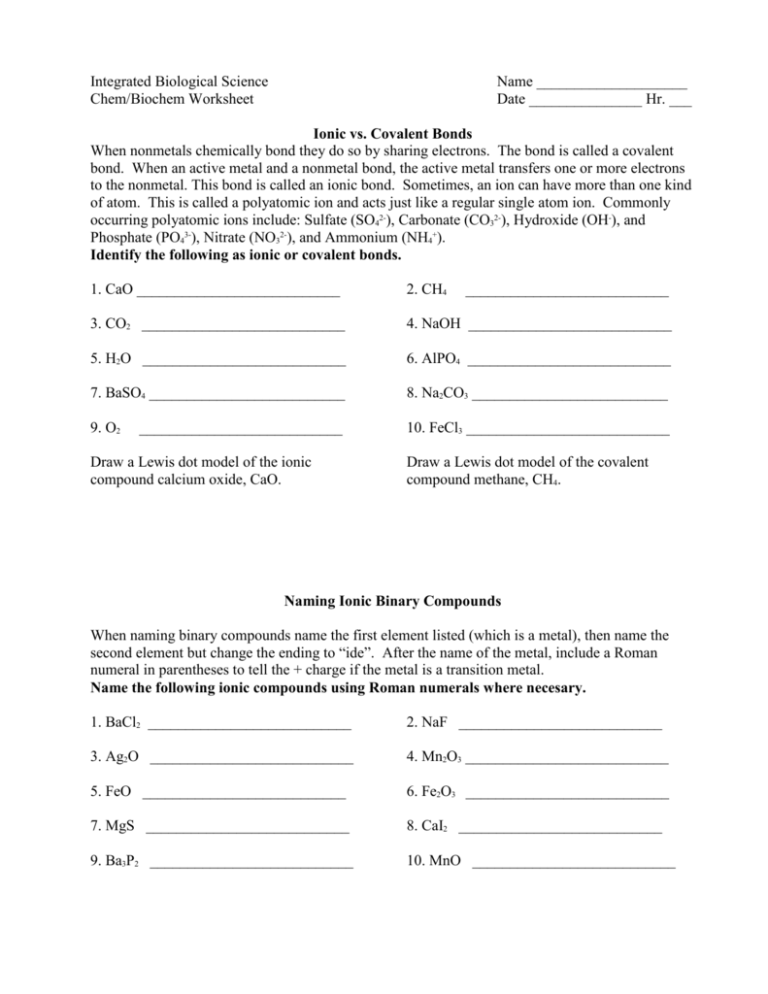
Integrated Biological Science Chem/Biochem Worksheet Name ____________________ Date _______________ Hr. ___ Ionic vs. Covalent Bonds When nonmetals chemically bond they do so by sharing electrons. The bond is called a covalent bond. When an active metal and a nonmetal bond, the active metal transfers one or more electrons to the nonmetal. This bond is called an ionic bond. Sometimes, an ion can have more than one kind of atom. This is called a polyatomic ion and acts just like a regular single atom ion. Commonly occurring polyatomic ions include: Sulfate (SO42-), Carbonate (CO32-), Hydroxide (OH-), and Phosphate (PO43-), Nitrate (NO32-), and Ammonium (NH4+). Identify the following as ionic or covalent bonds. 1. CaO ___________________________ 2. CH4 3. CO2 ___________________________ 4. NaOH ___________________________ 5. H2O ___________________________ 6. AlPO4 ___________________________ 7. BaSO4 __________________________ 8. Na2CO3 __________________________ 9. O2 10. FeCl3 ___________________________ ___________________________ Draw a Lewis dot model of the ionic compound calcium oxide, CaO. ___________________________ Draw a Lewis dot model of the covalent compound methane, CH4. Naming Ionic Binary Compounds When naming binary compounds name the first element listed (which is a metal), then name the second element but change the ending to “ide”. After the name of the metal, include a Roman numeral in parentheses to tell the + charge if the metal is a transition metal. Name the following ionic compounds using Roman numerals where necesary. 1. BaCl2 ___________________________ 2. NaF ___________________________ 3. Ag2O ___________________________ 4. Mn2O3 ___________________________ 5. FeO ___________________________ 6. Fe2O3 ___________________________ 7. MgS ___________________________ 8. CaI2 ___________________________ 9. Ba3P2 ___________________________ 10. MnO ___________________________ Naming Covalent Binary Compounds When naming covalent binary compounds (all nonmetals) just name the elements in the order they are listed but change the ending of the name of the last element to “ide”. Remember to use a prefix to tell how many of each element is present. 1. CO ___________________________ 2. CO2 ___________________________ 3. SO3 ___________________________ 4. N2O ___________________________ 5. CCl4 ___________________________ 6. P2O5 ___________________________ 7. CS2 ___________________________ 8. OF2 ___________________________ 9. PCl3 ___________________________ 10. N2O4 ___________________________ Naming Compounds (Mixed) If a polyatomic ion is included, the name of the ion is written without changing the ending. 1. NaCl ___________________________ 2. MnS ___________________________ 3. K2O ___________________________ 4. CO2 ___________________________ 5. PbSO4 ___________________________ 6. Li2CO3 __________________________ 7. NH3 ___________________________ 8. NO3 ___________________________ 9. Ca(OH)2 ___________________________ 10. AlPO4 ___________________________