III
advertisement

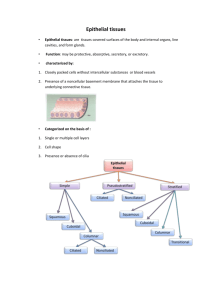
Digestive System III – Accessory glands Reading Assignment: Chapter 18 - Digestive System III: Liver, Gallbladder and Pancreas; pay special attention to Folders 18.1, 18.2 and 18.3 (Clinical Correlations) Outline: I. Extrinsic salivary glands II. Pancreas III. Liver A. Gall Bladder I. Salivary glands Recall that there are two sets of salivary glands: (1) Intrinsic located within the submucosa of the oral cavity and (2) extrinsic lying external to the oral cavity. Both sets of glands are named by their location (toponymy). The intrinsic salivary glands include the labial, lingual, buccal and palatal. All of the extrinsic salivary glands occur bilaterally and are named the parotid (para-otic), submandibular and sublingual. The salivary glands produce saliva. This secretion has a complex composition (see text Table 16.1) serving a variety of functions. In digestion, saliva moistens the food to assist in mastication, oral transport and deglutition; its dissolves taste stimuli permitting their binding to taste bud receptors, and its amylase initiates carbohydrate digestion. Saliva is also important in maintaining tooth enamel by re-mineralizing immature or carious enamel, neutralizing oral acids, and by forming the acquired pellicle, a thin film of salivary proteins that covers and protect the enamel. Finally, saliva has immune functions; its lysozymes destroy bacteria such as Staphylococcus and the salivary cells supply the secretory component of immunoglobulin A. The salivary glands are innervated by the autonomic nervous system. Stimulation by the sympathetic innervation produces a thick secretion high in organic content whereas stimulation by parasympathetic nerves produces a thin secretion low in organic content. Structure In contrast to the intrinsic salivary glands, the extrinsic salivary glands are encapsulated. In both groups of glands, septa formed by collagenous connective tissue divide the glands into lobes and lobules. Running through the septa are the vessels and nerves that supply the glands and the ducts which drain them. This connective tissue stroma is rich in lymphocytes and plasma cells (IgA) and also contains fibroblasts, mast cells and variable amounts of fat. The parenchyma of the salivary glands is formed by three types of exocrine gland cells (mucous, seromucous and serous) and myoepithelial cells. Serous cells are pyramidal in shape with a central nucleus. They are a typical protein secreting cell with a basophilic cytoplasm and secretory granules (eosinophilic) concentrated apically. These cells 2 synthesize and secrete amylase, secretory component (for IgA), lysozymes, and lactoferrin (an iron chelator). Mucous cells are cuboidal to columnar cells with a basal nucleus of condensed chromatin. As their name suggest, they synthesize and store mucus. The mucus is stored apically as mucinogen granules that stain poorly in paraffin sections. Seromucous cells are more common in the intrinsic salivary glands (e.g., lingual) and mucous glands of the respiratory system (e.g., trachea). They are intermediate in staining properties between mucous and serous cells with a slightly basophilic cytoplasm and poorly-staining mucinogen granules . They typically form secretory acini. Myoepithelial or basket cells are stellate-shaped cells with numerous processes. They lie within the basal lamina of the secretory epithelia and proximal duct. They contain contractile proteins that are used to move the glandular secretions from the end-piece lumen into the duct system. Release of the secretory granules by the salivary cells into the end-piece lumen is by exocytosis. The salivary glands are classically classified as racemous which is a fancy way of saying “full of clusters”. The clusters consist of exocrine cells that form secretory end-pieces which drain to a common duct. The shape of the secretory end-pieces are described as being acinar (spheroidal), tubular and tubulo-acinar. In general, the acinar end-pieces are usually comprised of serous or seromucous cells forming a serous acinus or seromucous acinus, respectively. In contrast, the tubules are usually comprised of mucous cells forming a mucous tubule. The tubulo-acinar end-pieces are typically mixed in composition taking the form of a mucous tubule with a crescent shaped cap of serous or seromucous cells. The entire structure is referred to as a mixed tubulo-acinus whereas the crescent shaped cap is referred to as the serous demilune or seromucous demilune, depending upon its composition. The unusual appearance of these mixed end-pieces is thought to be an artifact of formalin fixation. N.B. In the intrinsic salivary glands and the sublingual gland, the demilune is more often comprised of seromucous cells and is properly termed a seromucous demilune. Serous demilunes predominate only in the submandibular gland. Salivary ducts The salivary ducts not only convey the end-piece secretions to the oral cavity but also modify the secretion along the way. Starting from the secretory end-pieces, the duct system can be divided into 3 sequential segments. (1) The intercalated ducts lead directly from the end-pieces. They are comprised of a low cuboidal epithelium that secretes bicarbonate (to neutralize mouth acids) and absorbs chloride. The intercalated ducts feed into intralobular ducts which, as their names suggests, are typically found within the parenchymal lobes. The cells forming these ducts are cuboidal in shape. In the serous and mixed salivary glands (e.g, the parotid and submandibular glands) the intralobular ducts are also called striated ducts, named for their cell’s basal membrane interdigitations, an indication of ion transport. In this case, the striated ducts secrete potassium and absorb sodium. The intralobular ducts of the sublingual gland (primarily mucous) lack these striations and hence do not modify their lumenal contents. The intralobular ducts feed into the excretory ducts that connect to the oral cavity. These ducts are typically found within the septa and are also termed interlobular ducts. Little or no modification of the saliva is thought to occur in the excretory ducts. As the lumen of the excretory duct increase in diameter, the epithelium transitions from simple cuboidal to pseudostratified columnar or stratified cuboidal to stratified squamous at the duct opening. The extrinsic salivary glands The parotid gland is the largest of the three paired extrinsic salivary glands. It lies anterior to the external ear and its duct opens to the oral cavity opposite the second maxillary molar. Its capsule is poorly formed and is comprised of mainly serous acini interspersed with adipose tissue. 3 The submandibular gland is intermediate in size. It lies external to the oral cavity medial to the mandible in the molar region. Its duct opens at the anterior base of the tongue. It contains serous acini and mucous tubules as well as mixed tubulo-acini, the latter with serous demilunes. The sublingual is the smallest of the extrinsic salivary glands, lying beneath the anterior oral cavity. Its multiple ducts open to the floor of the oral cavity and it contains primarily mucous tubules and a few mixed tubulo-acin, the latter with seromucous demi-lunes. II. Pancreas The pancreas is an endocrine and exocrine glandular organ found deep to the transverse colon. Its expanded head rests in the C-loop of the duodenum and its body and tail extend towards the spleen. The pancreatic duct extends the length of the gland and empties into the hepatopancreatic ampulla. From the ampulla, pancreatic exocrine secretions (and bile) are released into the duodenum via the hepatopancreatic sphincter. The pancreas has a poorly developed connective tissue capsule. Septa extending from the capsule divide the gland into lobules. The endocrine and exocrine components of the gland are structurally distinct. The exocrine portion is comprised of serous glands (acinar or tubulo-acinar) that drain to duodenum via pancreatic ducts. These glands synthesize and secrete digestive enzymes. The endocrine cells are located in the pancreatic isles (islets of Langerhans) and synthesize and secrete the hormones insulin, glucagon and somatostatin to the systemic circulation. The serous cells of the exocrine glands are typical protein secreting cells. Pyramidal in shape, they have a basophilic cytoplasm with eosinophilic zymogen granules apically. They synthesize and secrete proteolytic enzymes (e.g., trypsinogen), lipases, nucleolytic enzymes (DNAse and RNAse) and amylase. Most of these enzymes are secreted as zymogens (proenzymes) and are activated only when they enter the duodenum. The duct system of the pancreas begins with the centroacinar cells that line the lumen of the acini. These are squamous cells with light cytoplasmic staining. The intercalated ducts are continuations of the centroacinar cells outside of the acini that feed into intralobular ducts (formed by a cuboidal epithelium) that in turn feed into interlobular ducts (formed by a low columnar epithelium). The intercalated ducts secrete a fluid rich in sodium and bicarbonate ions that will neutralize the acidic chyme of the duodenum and help establish the proper pH for enzyme activation. As in all exocrine ducts, as the pancreatic duct approaches its terminus, its lumen increases in diameter and its epithelium increases in stratification. Two hormones secreted by the enteroendocrine cells of the duodenum regulate the exocrine pancreas. The hormone secretin promotes release of a pancreatic fluid low in enzyme activity and high in bicarbonates. Thus, the hormone acts chiefly to stimulate the intercalated duct cells. Cholecystokinin (CKK) promotes release of a pancreatic fluid rich in enzymes and thus chiefly stimulates the serous cells. III. Liver The liver is both the largest glandular organ and the largest internal organ. Externally it is divided into 4 anatomical lobes that are of no physiological significance. Internally, the liver is organized into functional lobules that have no external expression. The porta hepatis is the hilum of the organ and serves as the entry of the hepatic arteries and portal vein and the exit of the hepatic ducts. The liver is covered by a thin connective tissue capsule. Functions 4 The functions of the liver are as numerous as the stars in the sky. These include: (1) Protein synthesis including serum proteins that regulate osmotic pressure (albumin, alpha and beta globulins), lipoproteins (HDLs, LDLs, VLDLs), and blood clotting agents (prothrombrin and fibrinogen). (2) Vitamin pathways: These include vitamin A (retinal) which is taken up, stored and released by the liver bound to retinal-binding protein (RBP); part of the Vitamin D synthetic pathway takes place in the liver; and Vitamin K is transported to the liver by chylomicrons, absorbed, used and released with VLDLs. (3) Iron storage and metabolism. (4) Processing of nutrients (carbohydrates, fatty acids, cholesterol, amino acids) (5) Endocrine function: Parts of Vitamin D, thyroxin and growth hormone pathways occur in liver; primary site of insulin and glucagons activity. (6) Exocrine function: synthesis and release of bile Bile Bile is produced by the hepatocytes; it is stored and concentrated in the gall bladder for eventual release to the duodenum. It is a complex mixture containing cholesterol, bile salts, bilirubin and electrolytes, each of which has separate fates. Cholesterol is absorbed in the gut and re-released by the liver for membrane and steroid synthesis in other cells. The bile salts act as emulsifying agents that promote fat digestion and absorption. Bilirubin is the end-product of hemoglobin degradation and is excreted along with the feces. The electrolytes serve to maintain the osmolarity of the bile on its journey to the duodenum. Blood supply The liver has a dual blood supply with both sets of vessels entering the porta hepatis. Nonsystemic venous blood from the portal vein (portal hepatic system) carries nutrients from the gastrointestinal tract and cell products from the pancreas (hormones) and spleen (blood breakdown products). The hepatic artery supplies oxygenated blood. Between the lobules vessels branching from the portal and arterial systems run with draining branches of the bile duct system to form portal triads (each triad consists of an interlobular bile duct, hepatic arteriole and portal venule). The terminal branches of the portal and arterial systems unite to form the hepatic sinusoids (= sinusoidal capillaries) which perfuse the hepatocytes. Ultimately, the hepatic sinusoids drain to the central canal (= terminal hepatic venule) and the blood returns to the caval (systemic) venous system via the hepatic vein. 5 Histological structure The parenchyma of the liver consists of plates (seen as cords in cross section) of hepatocytes one cell thick separated from each by the hepatic sinusoids (= sinusoidal capillaries). Between adjacent hepatocytes run the bile canaliculi (see below). The stroma consists of connective tissue which is continuous with the fibrous capsule and that serves as a conduit for vessels (vascular and lymphatic). The sinusoidal capillaries are endothelial lined vascular channels between the hepatocyte cords. Between the sinusoidal endothelium and the hepatocytes lies the peri-sinusoidal space (of Disse). This space is filled by villi from the hepatocytes that facilitate exchange of materials. Functional structure The functional organization of the liver can be viewed 3 ways depending upon which function is emphasized; the models are the (1) classic lobule, (2) portal lobule, and (3) liver acinus. The classic lobule emphasizes the flow of blood though the liver. The center of the lobules is the terminal hepatic venule (=central vein). This venule drains the sinusoidal capillaries which are fed by commingling branches of the portal vein and hepatic artery. Cords of hepatocytes radiate from the central vein to the perimeter of the lobule which are marked by the portal canals (= portal triads and their associated connective tissue. 6 The portal lobule emphasizes the exocrine function of the liver (i.e., bile secretion). The lobule consists of a bile duct and all the hepatocytes that contribute to it. The portal triad defines the center of the lobule (the end of the bile canaliculi) and the periphery is defined by the surrounding central veins (marking the start of the bile canaliculi). The liver acinus model is useful in explaining hepatocyte pathology. The acinus is an oval shaped unit whose short axis is defined by portal triads and whose long axis is defined by central veins. The hepatocytes are arranged into 3 concentric zones centered along the short axis: Zone 1 is closest to the blood supply, zone 2 is intermediate in distance and zone 3 is furthest from the blood supply and closest to the terminal hepatic venule (central vein). Zone 3 hepatocytes are the first to show pathological changes resulting from ischemia whereas zone 1 hepatocytes are the first to react to toxins or bile blockage (bile stasis). Cells The hepatocytes are the most abundant cell in the liver. These are the cells primarily involved in the exocrine, endocrine and metabolic functions of the organ. Roughly cuboidal in shape, they have an acidophilic cytoplasm and can be mono- or bi-nucleated. Arranged into plates (visible as cords in crosssection), each hepatocytes has 2 lumenal surfaces (facing the sinusoidal capillaries) and 4 hepatocyte/hepatocyte junctions. Each hepatocyte/hepatocyte contact contains tight junctions that created an intercellular lumen (1-2 um diameter) called the bile canaliculus which is the site of bile secretion. The lumenal surfaces lining the sinusoidal capillaries are covered with microvilli that project into the peri-sinusoidal space (of Disse). Electron microscopy of these cells reveals a cytoplasm abundant in both rough and smooth ER, peroxisomes (for de-toxification), Golgi units, mitochondria, and lipid and glycogen vesicles. The sinusoidal capillaries are lined by a discontinuous endothelium characterized by interendothelial gaps and large fenestra without diaphragms. The basal lamina beneath the endothelium is also discontinuous. These features combine to provide a very porous lining that allows for the free exchange of materials between the hepatocytes and blood serum. Stellate sinusoidal macrophages (Kupffer cells) are basically macrophages that form part of the sinusoidal lining. They may be important in the final degradation of erythrocyte by-products transported from the spleen. Hepatic stellate cells (Ito cells) are located in the peri-sinusoidal space [of Disse]. Their intracellular lipid vesicles are the primary storage site of vitamin A. Biliary tree The biliary tree is the system of conduits (ducts) through which bile flows. Starting from the hepatocytes they are as follows: bile canaliculi > intrahepatic ductules > interlobular ducts > hepatic ducts (L&R) > common hepatic duct > bile duct. All the names are descriptive or toponymic and follow the rules of anatomical nomenclature. The bile canaliculi lie between adjacent hepatocytes and their walls are formed by the tightly bound cell membranes of the hepatocytes. This is the site of bile secretion by the hepatocytes. The intrahepatic ductules are formed by several canaliculi just proximal to the triad; they are lined by a cuboidal epithelium. The interlobular bile ducts are the ducts of the portal triad. They receive bile from several intrahepatic ductules and their epithelium transitions from cuboidal to columnar with increasing lumen diameter. The hepatic ducts (L&R) are fed by interlobular bile ducts and emerge from the liver to form the common hepatic duct. When the common hepatic duct is joined by the cystic duct from the gall bladder, the combined ducts become the bile duct. The bile duct terminates in common with the 7 pancreatic duct in the hepatopancreatic ampulla. Bile is released to the ampulla by the bile duct sphincter and in turn the hepatopancreatic sphincter regulates release of ampulla contents into the duodenum. A. Gall bladder [vesica fellea] The gall bladder is a distensible storage sac (volume ~50 ml) attached to the visceral surface of the liver. It connects to the bile duct via the cystic duct. This blind pouch receives dilute bile from the liver and concentrates it via water removal by a factor of x10. The presence of fat in the lumen of the duodenum signals enteroendocrine cells to secrete cholecystokinin (CKK) causing the muscular wall of the gall bladder to contract and release bile. The wall of the gall bladder is composed of 3 layers: A mucosa (epithelium and lamina propria), muscularis and adventitia/serosa. The epithelium of the mucosa is a simple columnar epithelium which forms plica (folds) to increase surface area. These cells are called [it’s a tongue-twister) cholecystocytes and microvilli cover the apical surface further increasing surface area. Junctional complexes between adjacent epithelial cells tightly seals off the lumen and lateral folds increase the surface area between cells. A variety of ions (Na+, Cl-, HCO3-) are actively transported from the lumen into the lateral intercellular spaces. The resulting osmotic gradient draws water from the lumen thus concentrating the bile. The lamina propria lacks lymphatic vessels but is rich in fenestrated capillaries, lymphocytes and plasma cells. The muscularis is a thick layer of smooth muscle bundles arranged randomly (typical of detrussor muscles) and intermingled with collagen and elastic files. Contraction of this muscle causes release of the concentrated bile into the cystic duct. The adventitia / serosa are a dense connective tissue containing normal constituents. 8 9