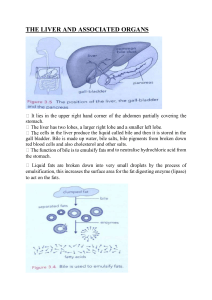
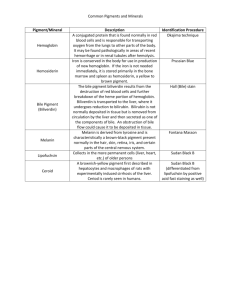
INSTRUCTION for medical application of HEPABEL Composition of
advertisement

INSTRUCTION for medical application of HEPABEL Composition of the medicine: Active ingredient: 1 tablet contains Artichoke (Cynara scolymus) dry extract 200 mg (equivalent 1,4 mg chlorogenic acid); excipients: potato starch, purified siliceous earth, dimeticone, calcium hydrogen phosphate dihydrate, talc, hydrogenated castor oil, silica colloidal anhydrous, magnesium stearate. Coating: povidone, titanium dioxide (E171), OPADRY OY-L-28900 (lactose monohydrate, hypromellose (E 464), titanium dioxide (E171), polyethyleneglycol 4000). Pharmaceutical form. Film-coated tablets. Main physicochemical properties: round and biconvex tablets, with white filmcoating. Name and address of manufacturer. BRUPHARMEXPORT s.p.r.l. 14 rue de la Grotte, 1020 Brussels, Belgium. Pharmacotherapeutic group. Alimentary tract metabolism. Bile therapy. Code ATC: A05A X10**. Pharmacological properties. Hepabel – is a plant preparation. Its pharmacological properties are associated with multiple active substances as follow: cynarin, caffeic acid and chlorogenic phenol acids, bioflavonoids, glycosides, phytosterols, terpenoids, carotene, vitamins, inuline and enzymes. All the substances are parts of Сynara scolymus leaves. Hepabel has choleretic, hepatoprotective, antioxidant, hypocholesteremic, diuretic and detoxifying effects. Hypocholesteremic action of Hepabel associated with 3-hydroxy-3-methylglutaryl-CoA – reductase inhibition. Cynarin with phenol acides has choleretic effect (by increasing bile volume and bile salts secretion), also it prevents cholestasis in bile passages, intensifies pancreatic enzymes secretion. Hepabel takes antioxidant effect, decreases lipid peroxydation and malonic dialdehyde level. Hepatoprotective effect associated with hepatocyte membranes stabilisation. Indications. - Dyskinesia of bilious ways (hypokinetic type); - chronic cholecystitis; - chronic hepatitis; - liver cirrhosis; - chronic renal insufficiency; - chronic nephritis; - chronic intoxications (hepatoxic substances, nitrocompounds, alkaloids, salts of heavy metals). Contra-indications. Allergy to one of the products components and Asteraceae family plants. Any bile duct obstruction. Severe disorders in the liver. Cholelithiasis, acute diseases of kidneys and liver. Acute diseases of bilious ways and urinary tracts. Special warnings and special precautions for use. It is necessary to appoint with care to patients with a diabetes. It is necessary to support a diet usually recommended to patients with diseases of a liver. To avoid the use of fat food, eggs, chocolate. - Pregnancy and lactation. Due to a lack of information, women are advised not to use Hepabel during pregnancy and lactation. - Effects on ability to drive and use machines. None. - Children. To not apply at children till 12 years. Methods of administration and dosage. Adults: 1 tablet 3 times a day, 20 minutes before meal. Children’s older than 12 years: 1 tablet 2 times a day, 20 minutes before meal. Course of treatment is 10-20 days, can be repeated if required in 1-2 months. Overdose. Not registered. Side effects. Hepabel can have a laxative effect in some rare cases. Interactions with other drugs. Not observed. Shelf-life. 3 years. Storage conditions. Store away from light at a temperature below 25ºC. Keep out of reach of children. Package. Containers of 60 film-coated tablets. Delivery conditions. Without prescription.