Name_____________________________________ Date____________ Period___ Combine Gas Law Problems
advertisement

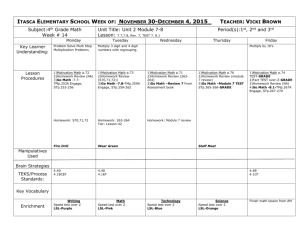
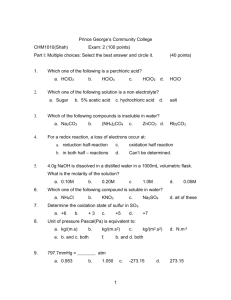
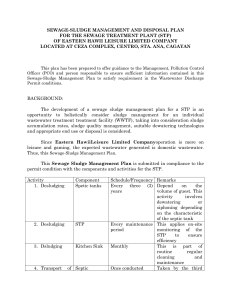
Name_____________________________________ Date____________ Period___ Combine Gas Law Problems STP = Standard Temperature and Pressure: T - 0°C P - 1 atm or 760 torr or 101.3kPa 1. What will the final pressure of a gas that expands from 1L at 10°C to 10L at 100°C, if the original pressure was 3atm? 2. A sample of Argon (Ar) gas occupies 50L at standard temperature and pressure. Assuming constant pressure, what volume will the gas occupy in the temperature is doubled? 3. For the graph below identify the two variables involved. Which is independent and which is dependent? 4. If the absolute temperature of 2L of gas at constant pressure changed from 283.15K to 566.30K, what would be the final volume? 5. A 35L sample of Hydrogen gas at STP is heated to a temperature of 55°C and a pressure of 3040torr. What is the volume of the gas under these new conditions?