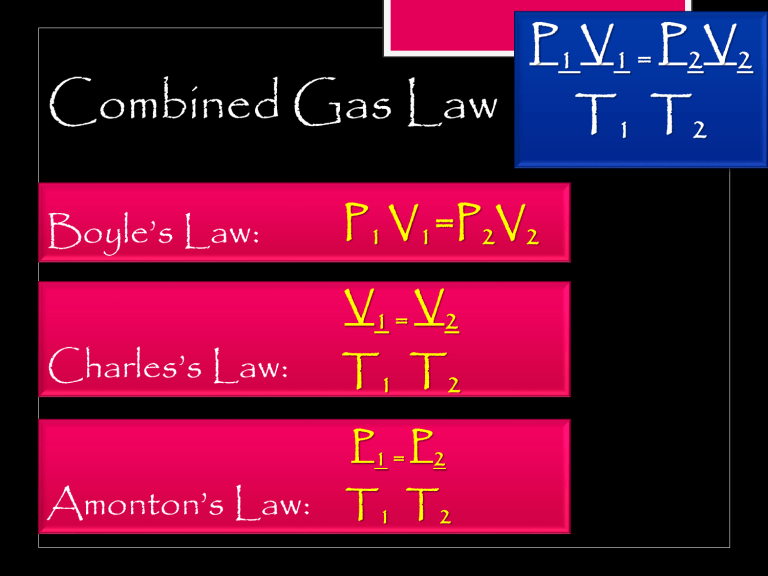
Combined Gas Law Boyle’s Law: Charles’s Law: P 1 V1 = P 2V2 P1 V1=P2V2 V1 = V2 T1 T2 P1 = P2 Amonton’s Law: T1 T2 T1 T2 P 1 V 1 = P 2V 2 T1 T2 STANDARD TEMPERATURE AND PRESSURE standard set of conditions for experimental measurement to enable computations between sets of data investigated the relationship between pressure and temperature in gases T: 273 K P: 760 mmHg - International Union Of Pure And Applied Chemistry (IUPAC) STANDARD TEMPERATURE AND PRESSURE T = OºC (273 K) P = 1 atm (760 mm Hg) SAMPLE PROBLEM #1: A 12.01 L sample of air is at STP. What would be its volume if its pressure is decreased to 500 mm Hg and its temperature is doubled? SAMPLE PROBLEM #2: A 200 L container at STP was crushed thereby reducing its size to one-fourth its volume and increasing its pressure to 5.00 atm. How hot is air in the container in ºC Seat Work Practice Exercises (1-3) Page 259