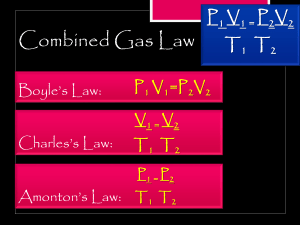
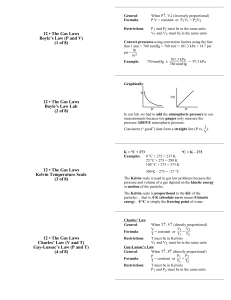
PAP Notes For Gas Laws Boyle’s Law: At a constant temperature and constant amount of gas, PRESSURE and VOLUME are inversely proportional to one another. P1V1 = P2V2 Charles’ Law: At a constant pressure and constant amount of gas, TEMPERATURE and VOLUME are directly proportional to one another. V1/T1 = V2/T2 Gay-Lussac’s Law: At a constant volume and constant amount of gas, TEMPERATURE and PRESSURE are directly proportional to one another. P1/T1 = P2/T2 Avogadro’s Law: At a constant temperature and constant pressure, VOLUME and the NUMBER OF MOLES OF GAS are directly proportional to one another. V1/n1 = V2/n2 Combined Gas Law: Combines Boyle’s, Charles’, and Gay-Lussac’s laws into one expression. With this equation we can see how changing MORE THAN ONE VARIABLE affects our unknown. P1V1/T1 = P2V2/T2 Ideal Gas Law: An ideal gas must follow the Kinetic Molecular Theory of Gases. We have talked about four variables that affect the behavior of gases. The four gas variables are: PRESSURE, VOLUME, TEMPERATURE, and NUMBER OF MOLES OF GAS. If we know 3 of the 4 variables, we can use the ideal gas law equation to solve for the unknown. Lastly, the constant in the equation shown below is R, known as the the ideal gas constant, and it will always be the same. PV = nRT DaIton’s Law of Partial Pressure: The TOTAL GAS PRESSURE is equal to the SUM OF ALL OF THE INDIVIDUAL PRESSURES of each gas. This is only absolutely true for ideal gases, but the error is small for real gases. An ideal gas must follow the Kinetic Molecular Theory of Gases. PT = P 1 + P2 + P 3 + … Factor Variable Units Useful Conversions atm 1 atm = 760 mmHg Torr Pressure P 1 atm = 760 Torr Pa 1 atm = 101326 Pa kPa 1 atm = 101.326 kPa mmHg 1 L = 1000 mL L Volume V 1 L = 0.001 m3 mL or m3 1000 L = 1 m3 Moles n moles n = number of moles of substance Temperature T K K = °C + 273.15 Values of R: Ideal Gas Constant R (see “Values of R” in the next column) * 0.0821 L•atm/mol•K 8.3145 L•kPa/mol•K 62.364 L•mmHg/mol•K * So, which value of R should I use? Because of the various values of R you can use to solve a problem, it is crucial to match your units of pressure, volume, temperature, and number of moles with the units of R. Standard Temperature and Pressure (STP): Sometimes word problems will tell you that you are “at standard conditions” or “at STP.” This means standard conditions of temperature and pressure. Here’s what you should know: The value of STP is 1 atm for pressure and 0°C for temperature. You can convert pressure into other units: P= 1 atm = 760 mmHg = 760 torr = 101.3 kPa Note that you always need to use Kelvin for temperature, so the standard temperature should be 273.15 K. T= 273.15 K At STP, 1 mole of gas will take up 22.4 L of volume.