– Unit 11 Worksheet 3 General Chemistry
advertisement

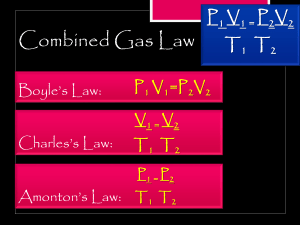
Name ___________________________________ Date _________________ Pd_____ General Chemistry – Unit 11 Worksheet 3 Useful Equations and Equalities: PV = nRT 1.0 atm = 760 mmHG = 101.3 kPa STP – T=273 and P=1.0 atm R = 0.0821 L x atm/ K x mol K = ْC + 273 1. A sample of argon gas is at a pressure of 1.24 x 104 kPa and a temperature of 24 ْC in a rigid 25-L tank. How many moles of argon gas does this tank contain? 2. A 35.0 L tank contains 7.00 moles of compressed air. The pressure inside the tank is 2400 mmHg, what is the temperature of the compressed gas? 3. How many grams of helium does a 25.0 L balloon contain at 0.850 atm and 22 ْC? 4. Calculate the volume that 2.25 moles of oxygen gas will occupy at STP. 1 5. A sample of water vapor occupies a volume of 10.5 L at 200.0 ْC and 1.2 atm. What volume will the water vapor occupy when it is cooled to 27ْC if the pressure remains constant? 6. Magnesium metal reacts vigorously with hydrochloric acid to produce hydrogen gas and magnesium chloride. What volume of hydrogen gas should be produced at 25 ْC and 0.950 atm if 2.55 grams of magnesium reacts completely with an excess of hydrochloric acid? 7. What mass of nitrogen gas do you have if you have a volume of 4.72 L at 26 ْC and 734 mmHg? 8. What is the volume of 35.0 g of carbon dioxide gas at STP? 2